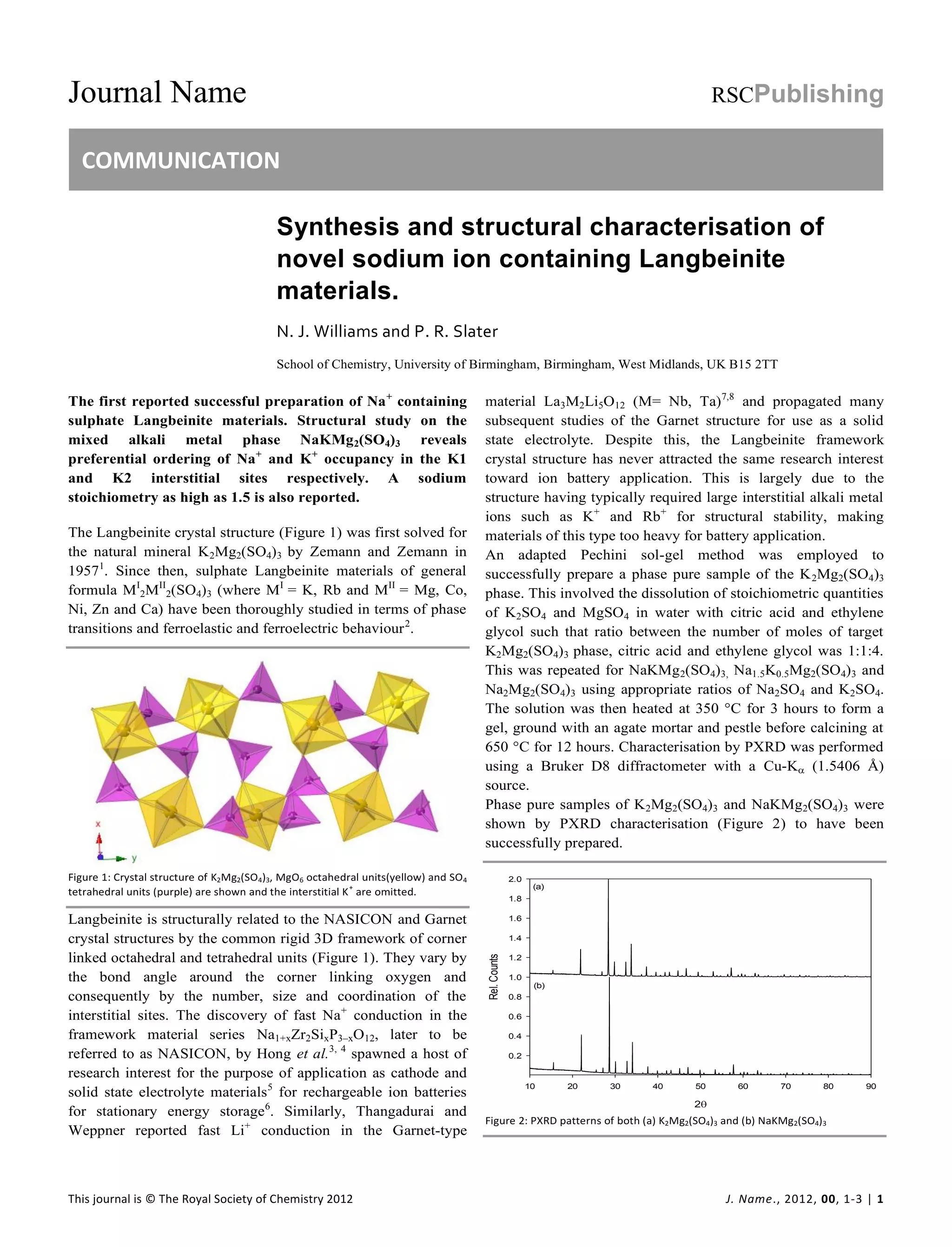
This document reports on the successful synthesis of novel sodium-containing Langbeinite materials through a sol-gel method. Structural characterization revealed that sodium preferentially occupies the larger interstitial sites, while potassium occupies the smaller sites. Higher sodium stoichiometries were achieved than previously reported. This discovery opens up the possibility of using Langbeinite structures in sodium ion batteries.