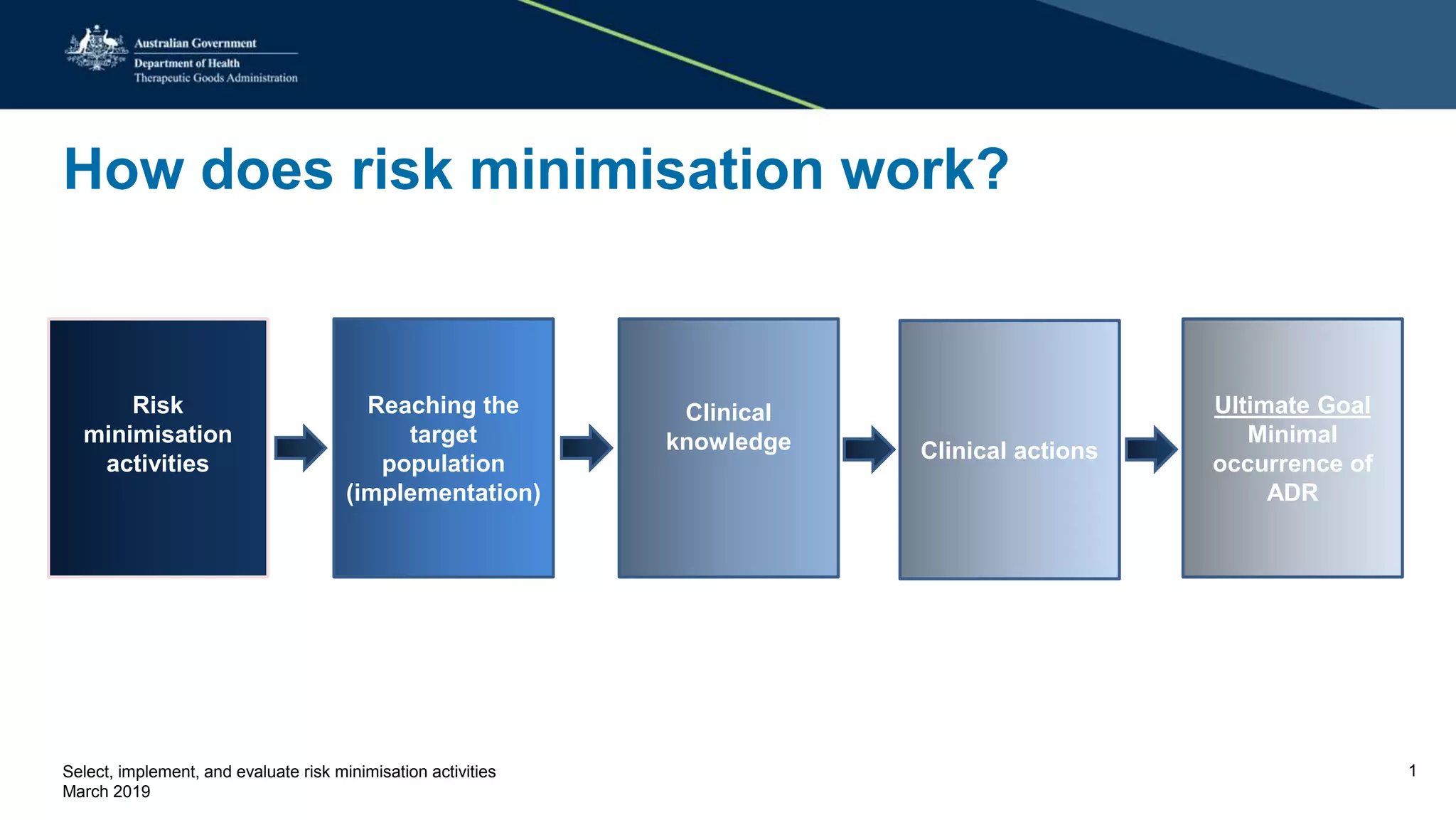
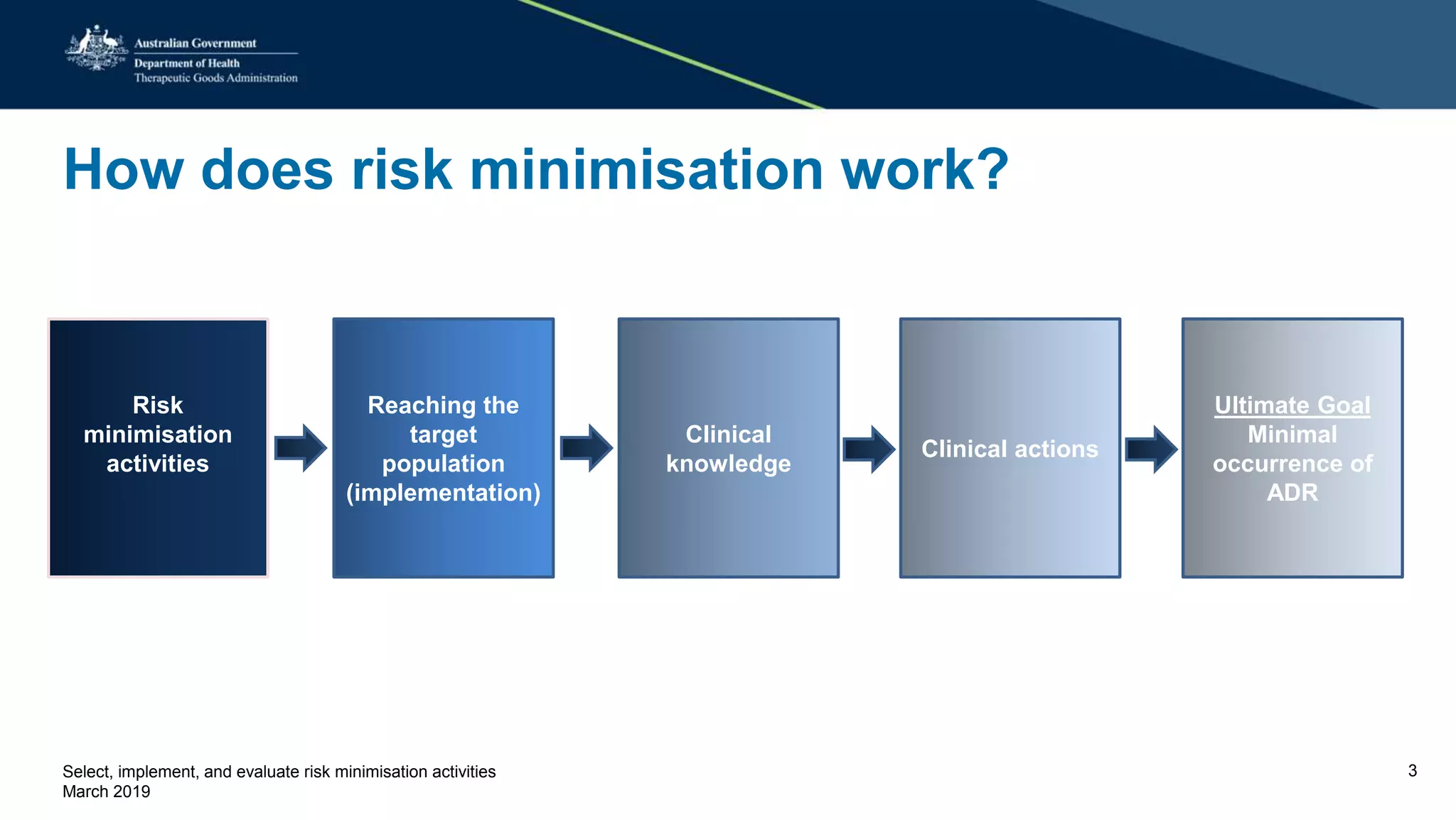
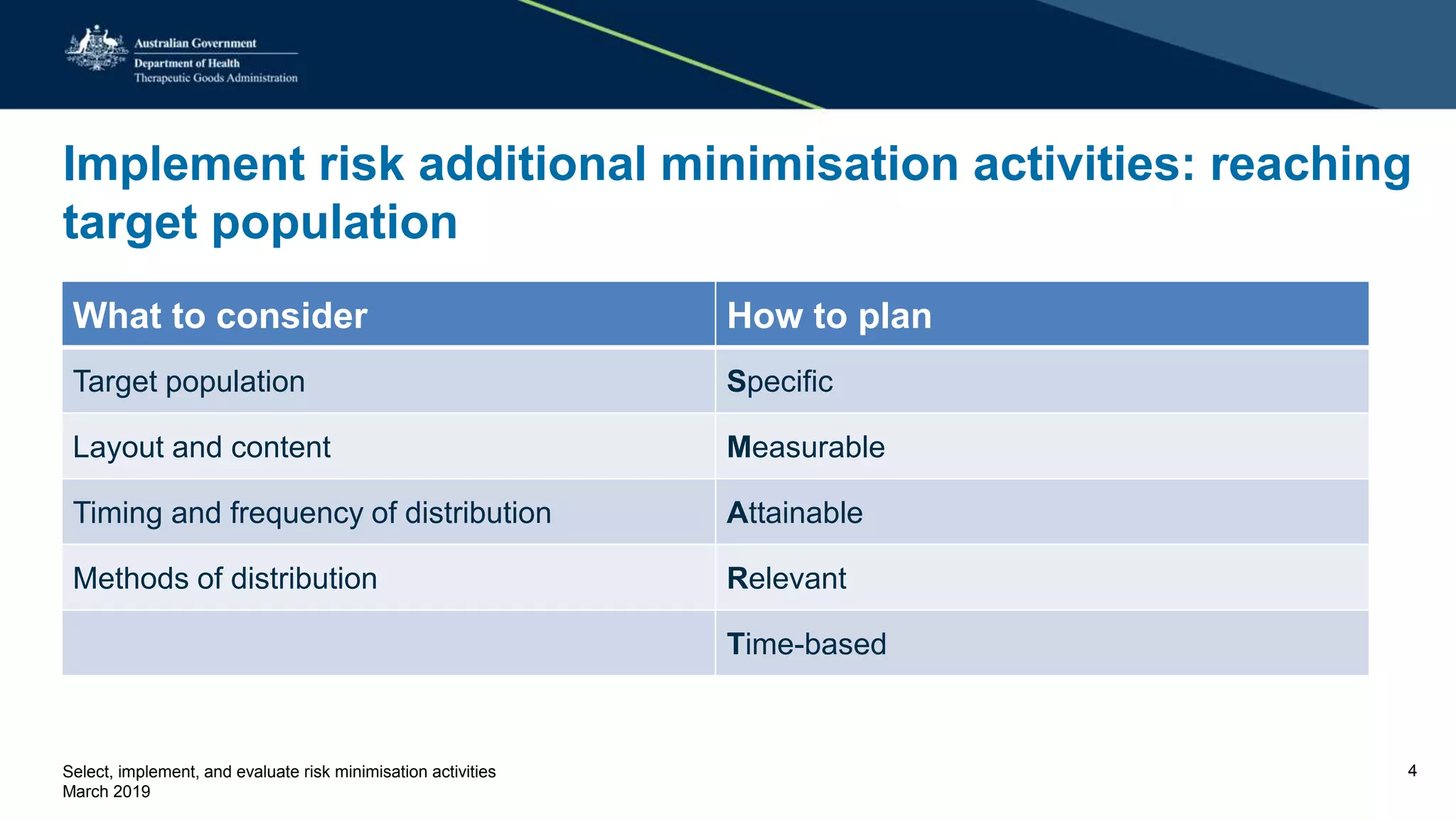
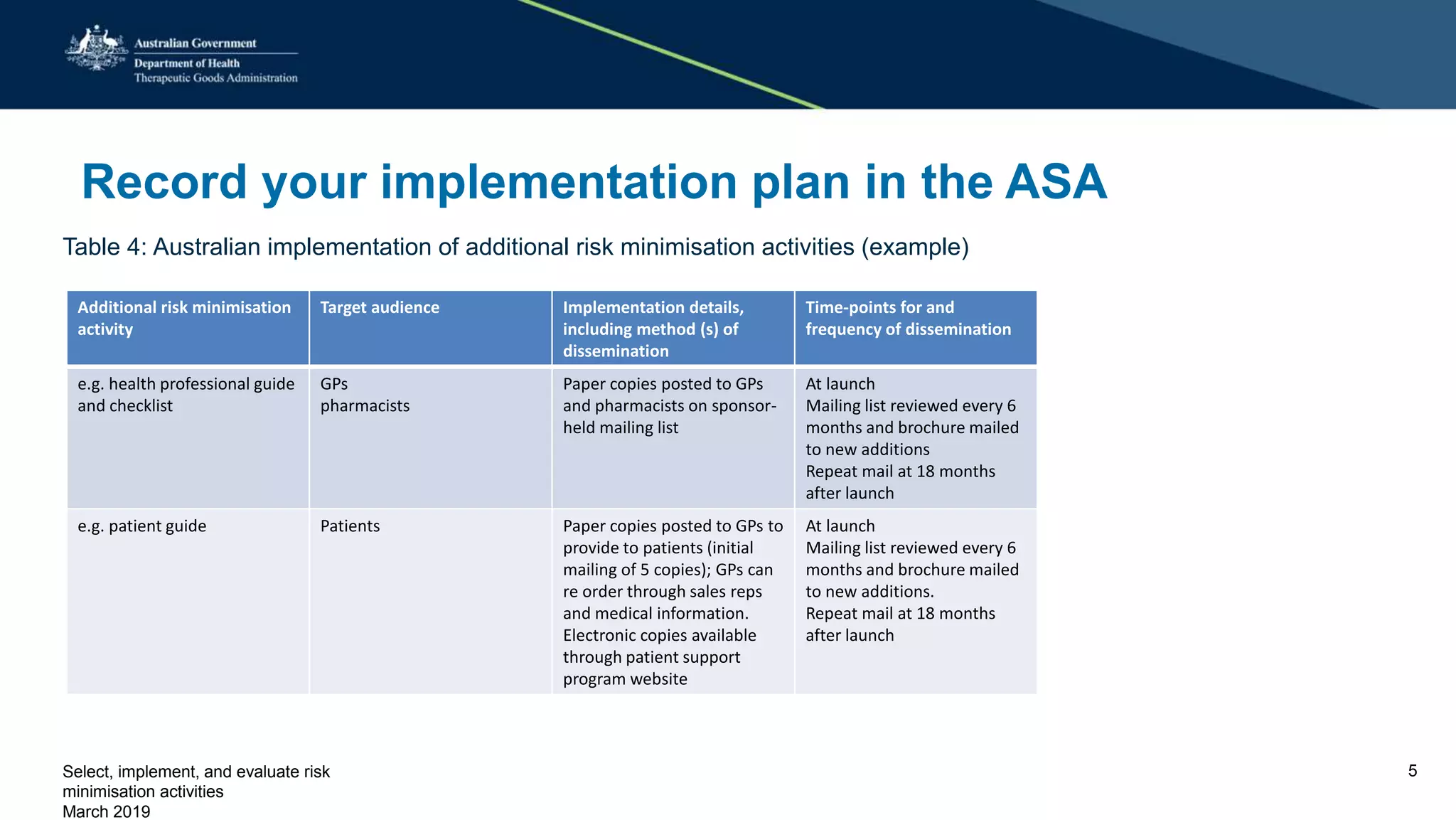
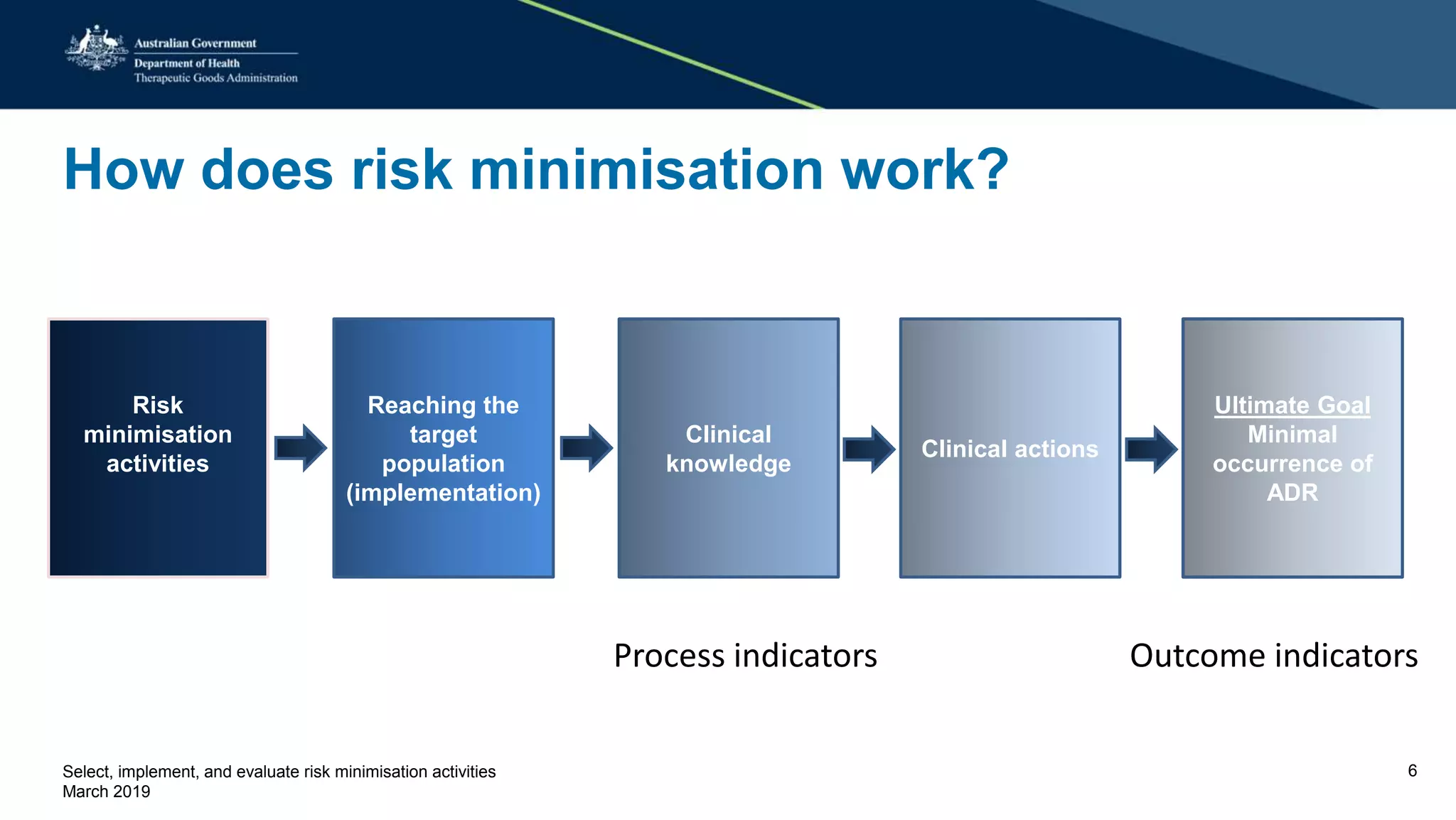
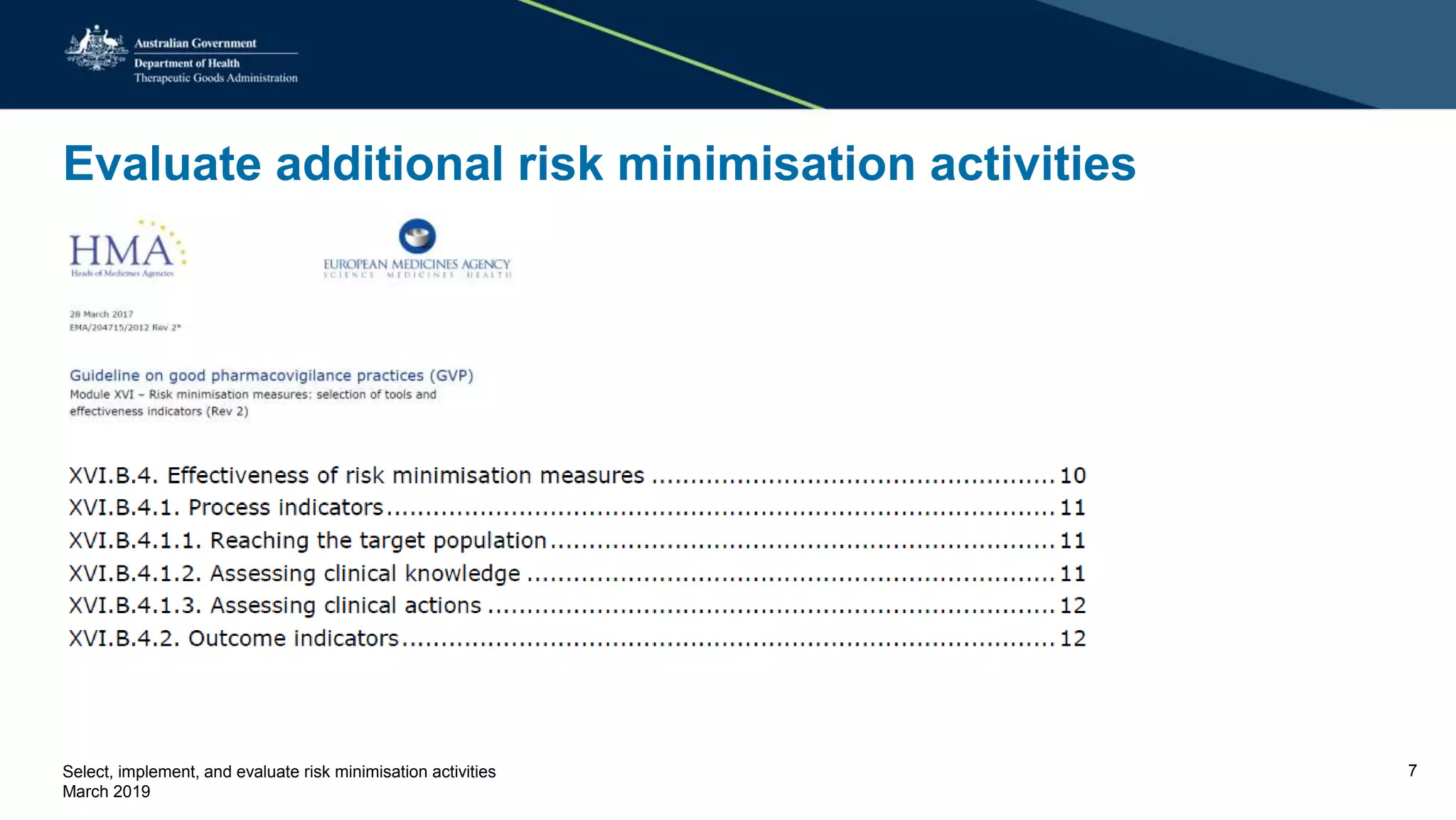
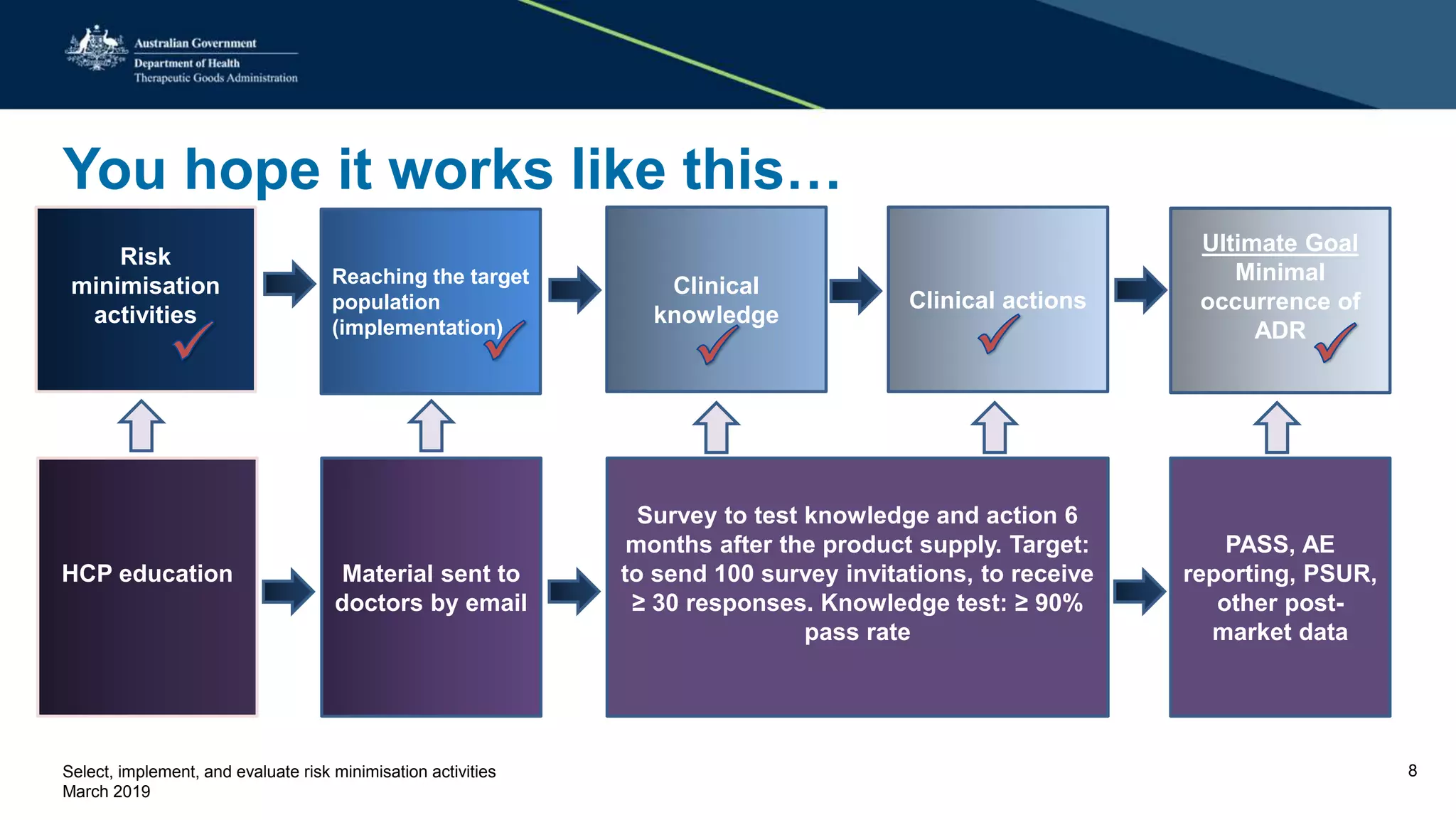
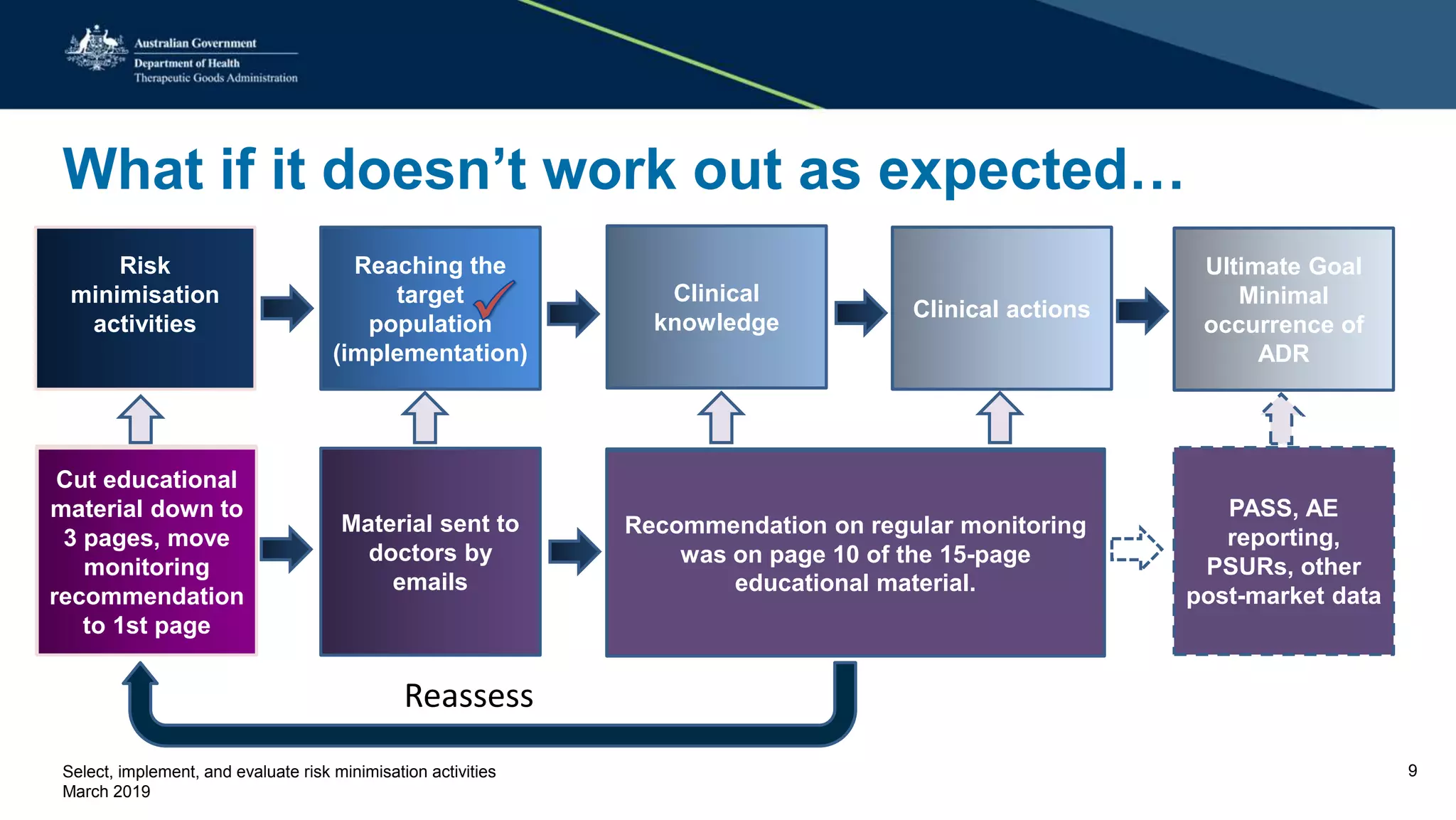
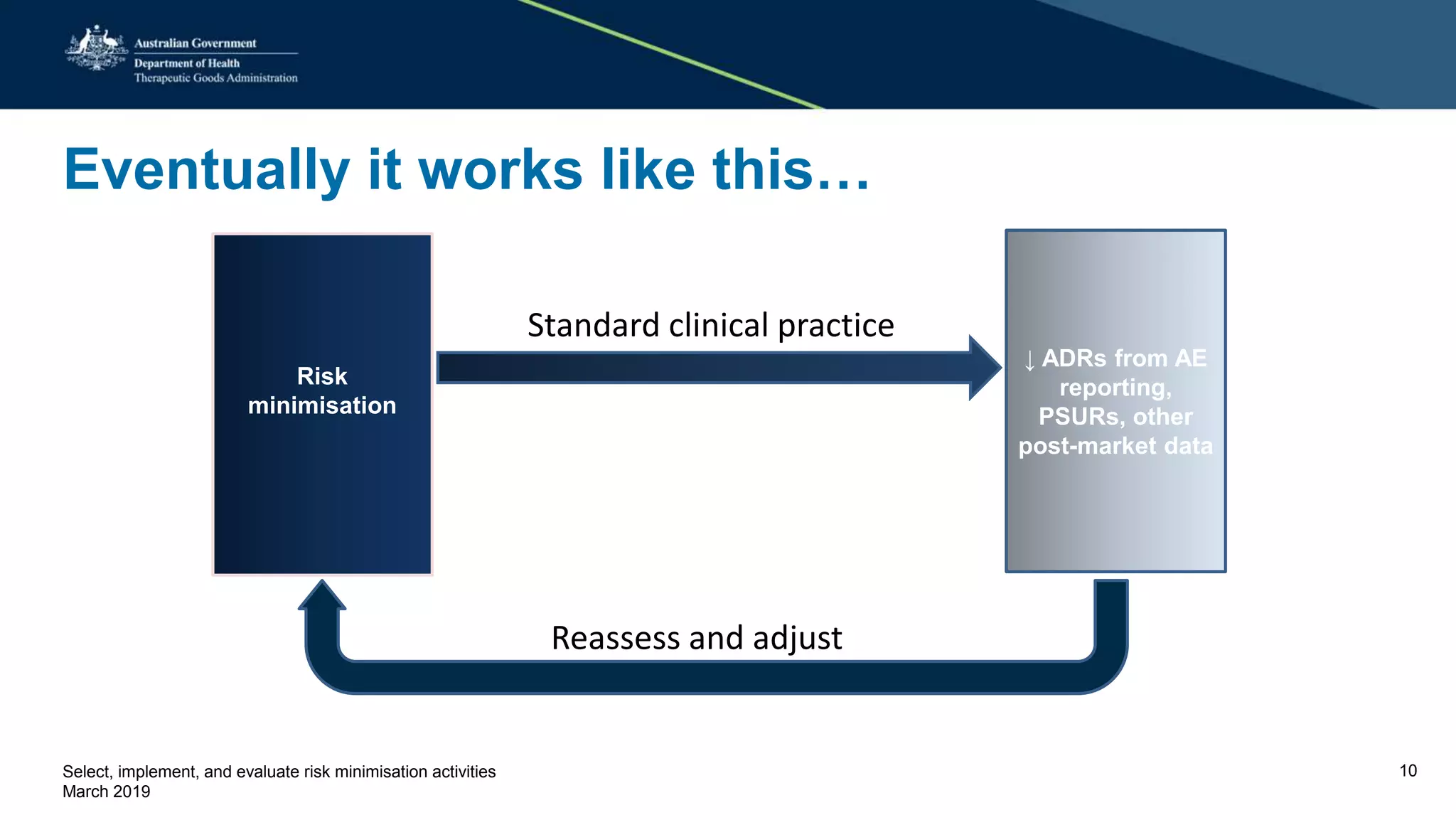
This document discusses risk minimization activities for pharmaceutical products. It explains that risk minimization involves selecting, implementing, and evaluating activities to reach the target population and minimize adverse drug reactions. Key steps include selecting additional risk minimization measures, implementing them by disseminating information to the target groups, and evaluating the implementation through process and outcome indicators with the goal of minimizing adverse drug reactions. The document provides examples of implementation plans and evaluating effectiveness through surveys. It notes that risk minimization activities may need to be reassessed and adjusted if not working as intended.