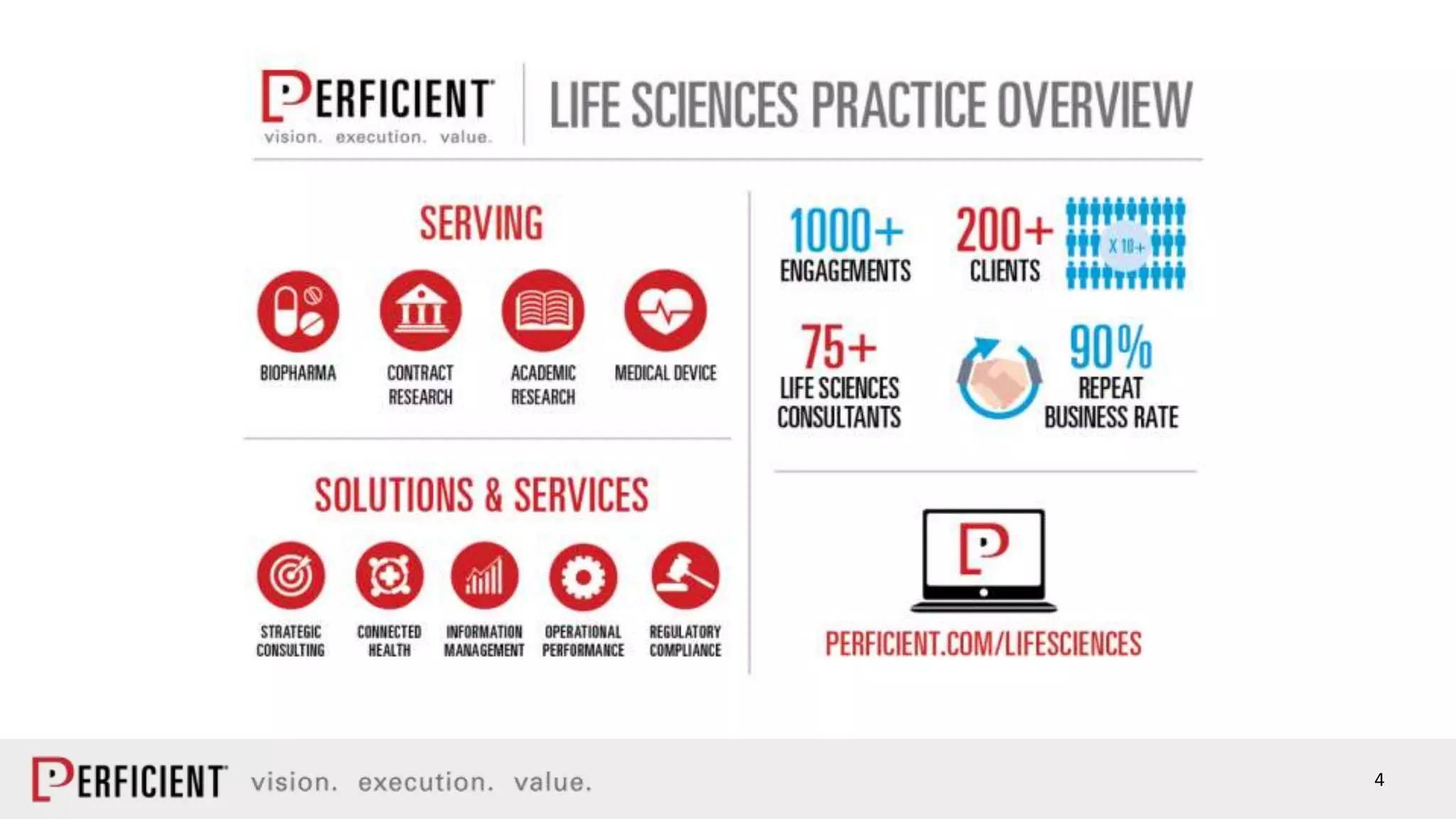
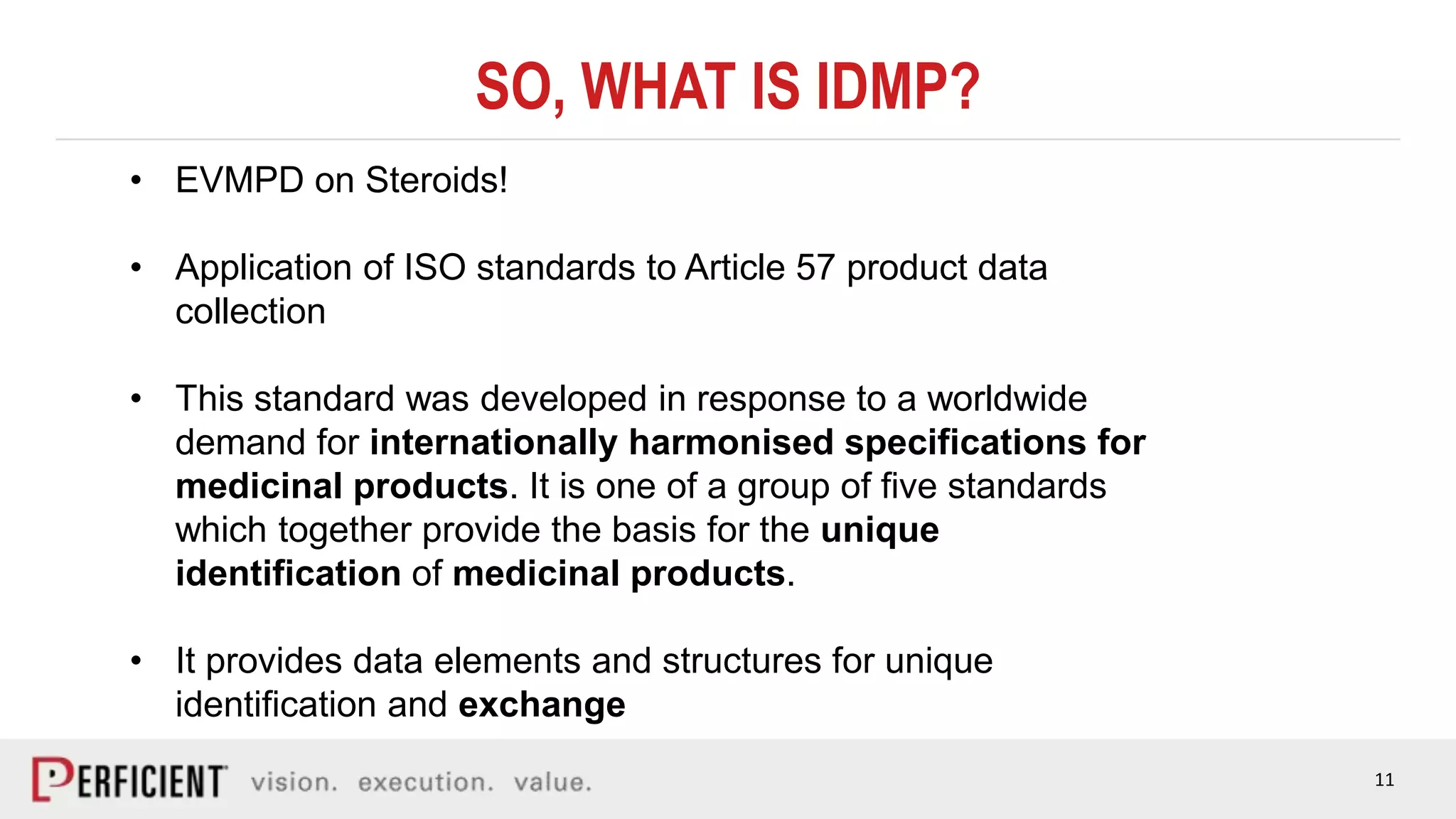
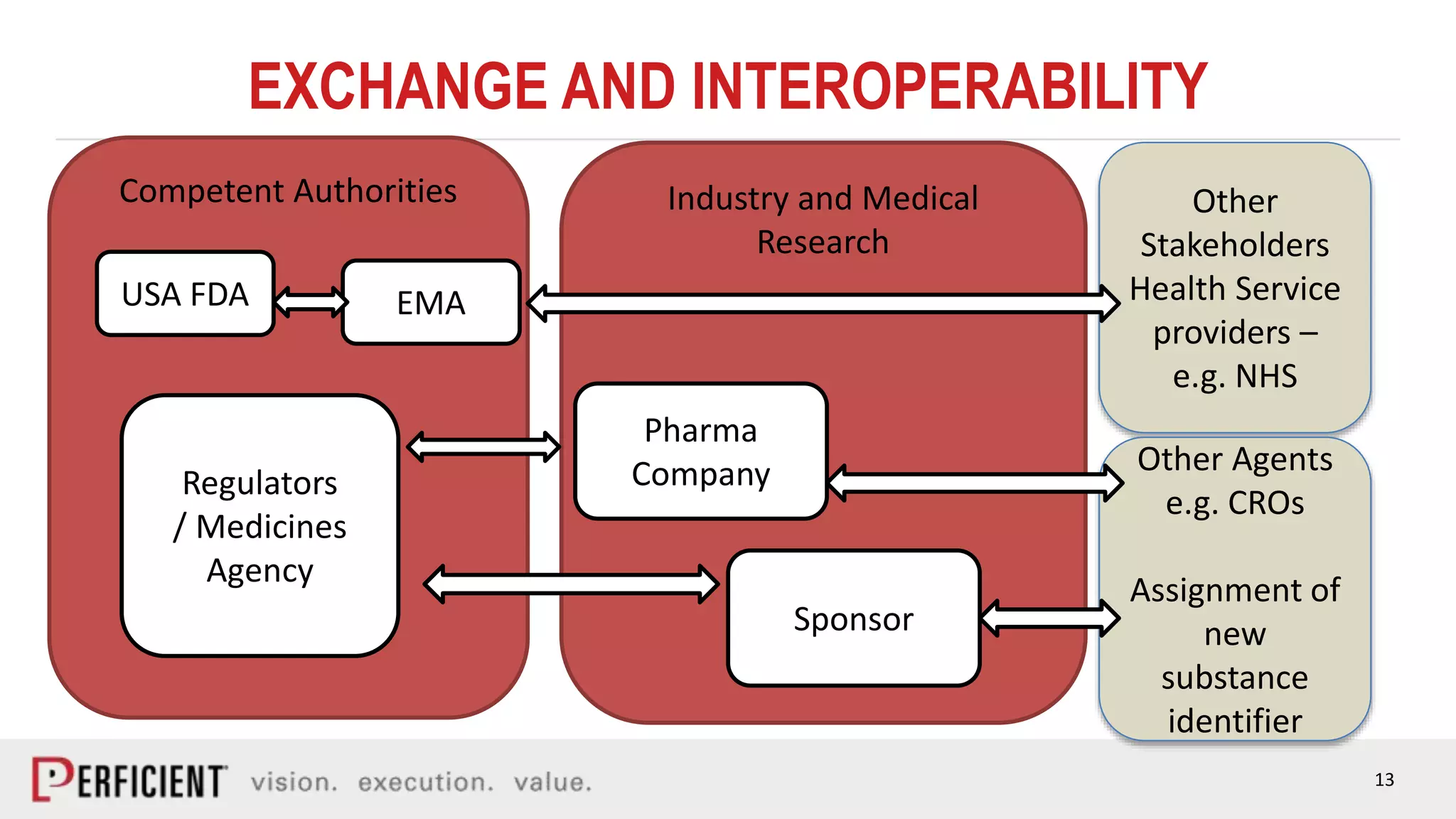
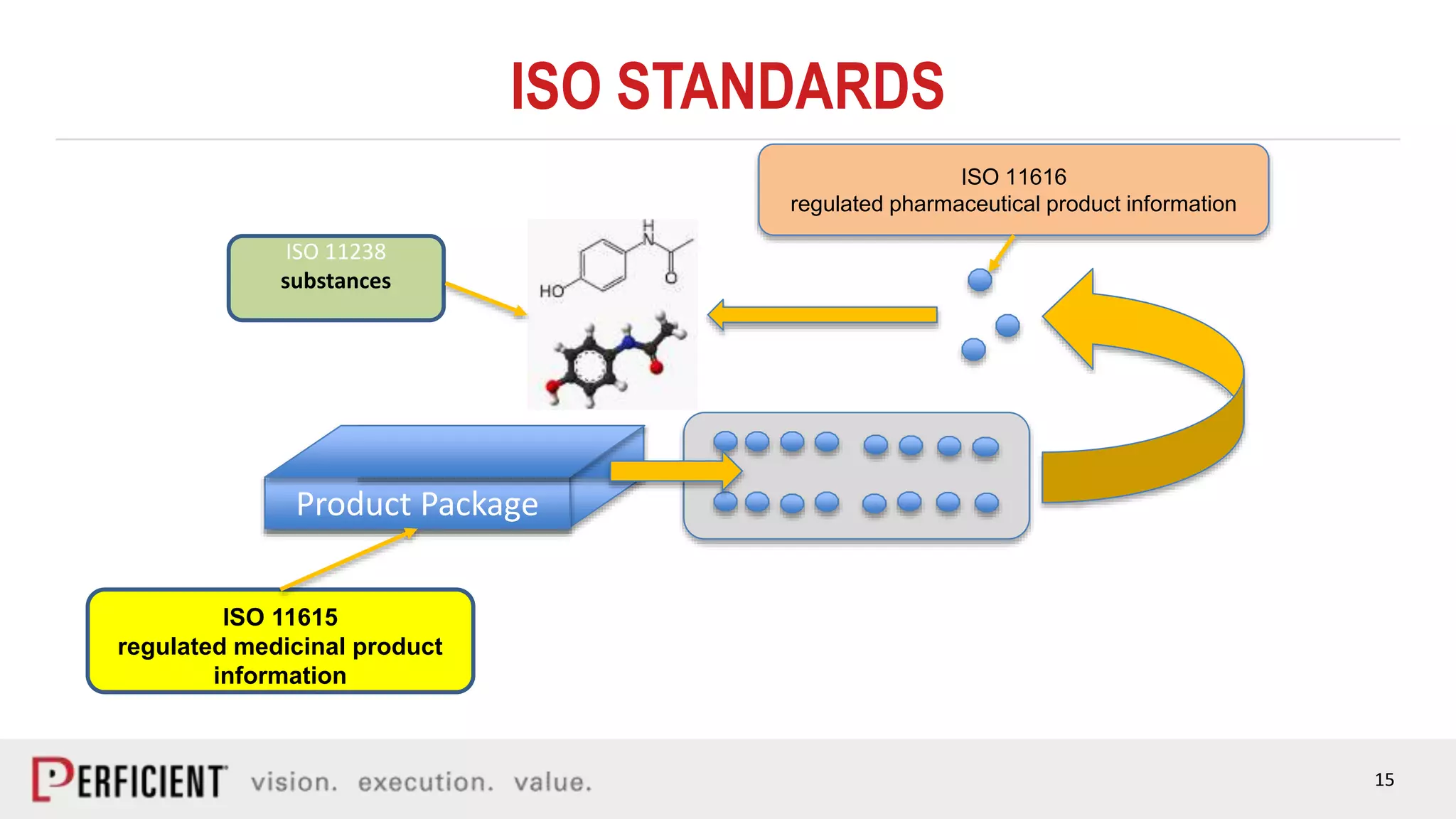
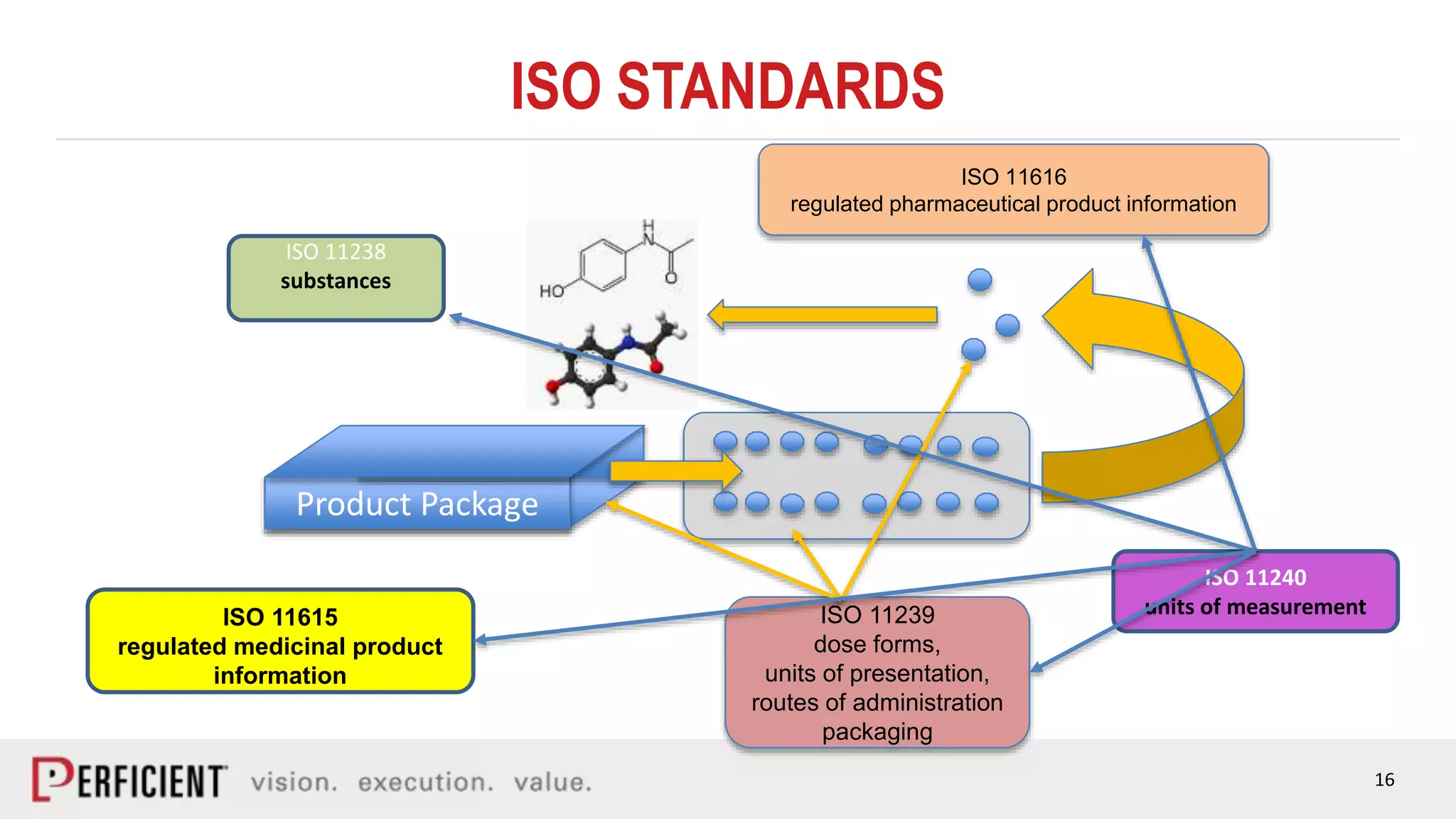
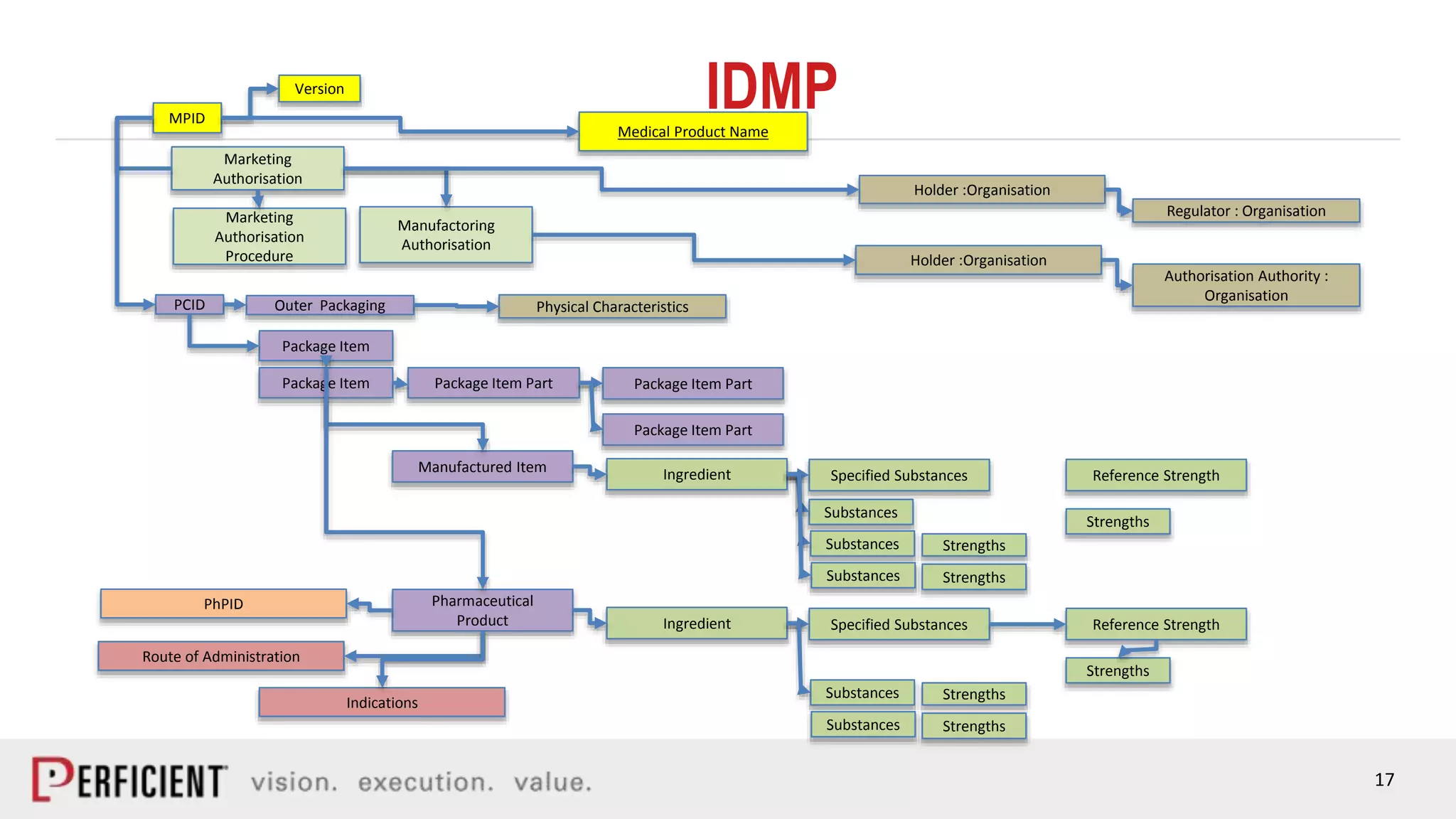
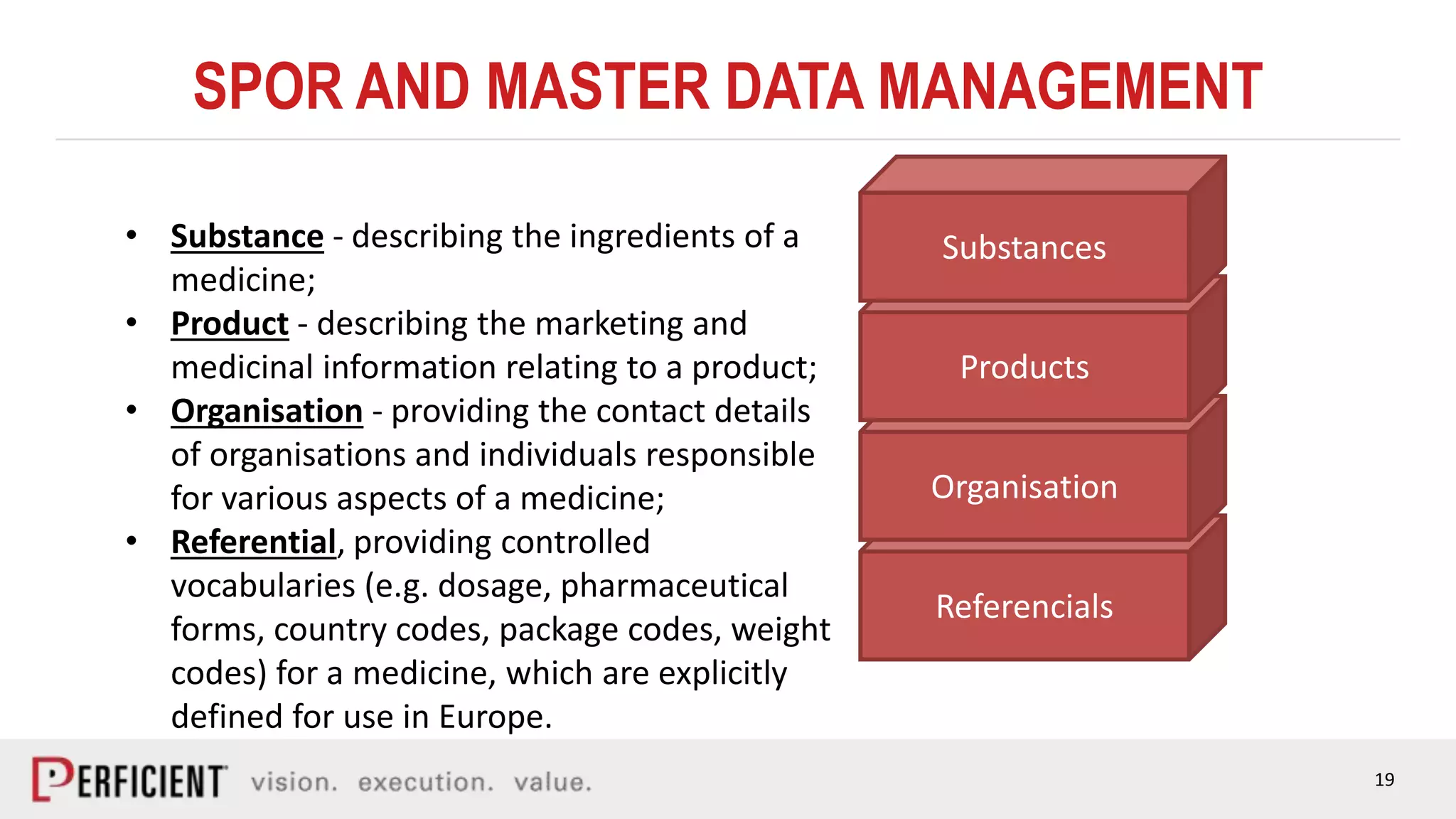
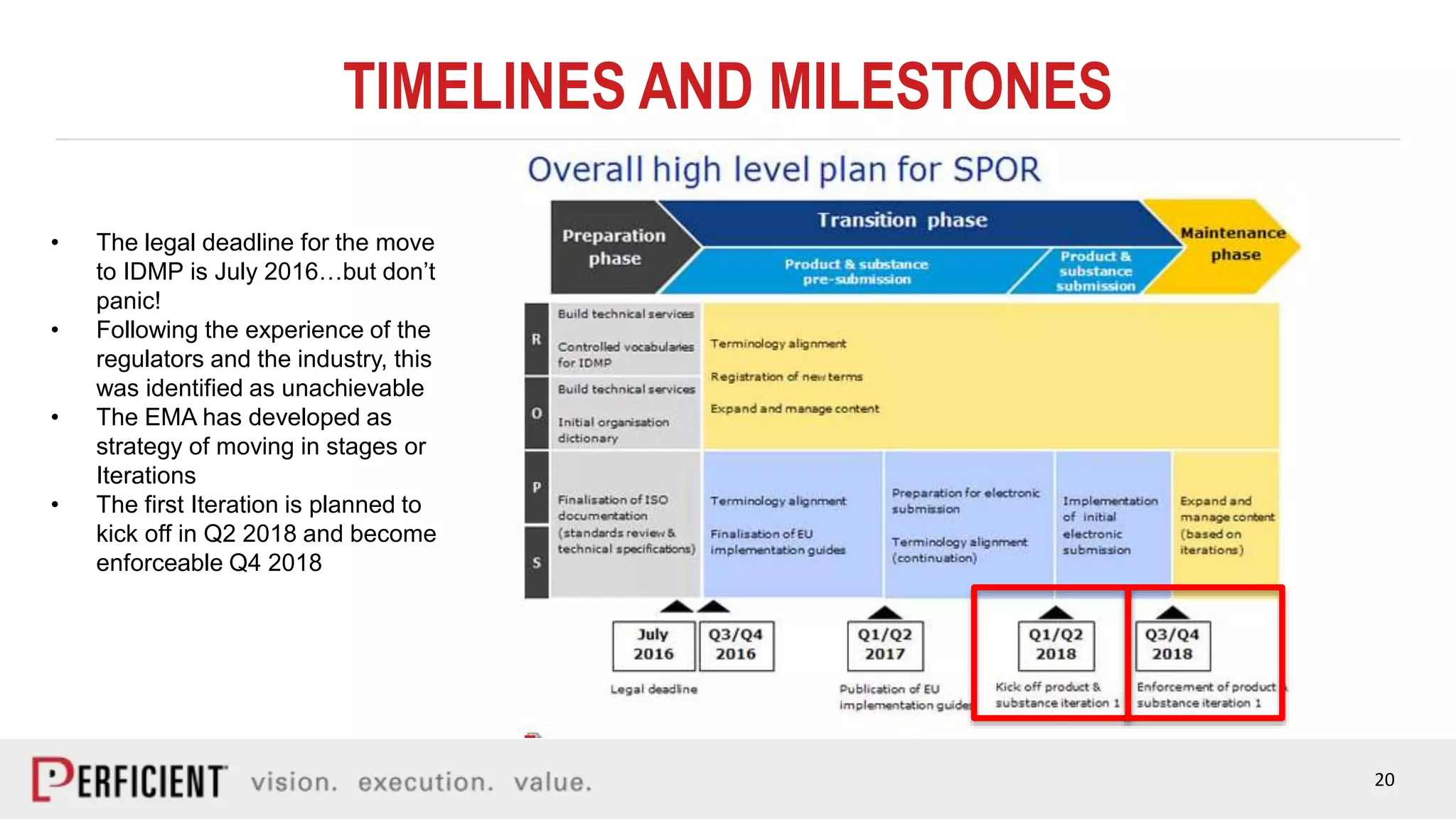
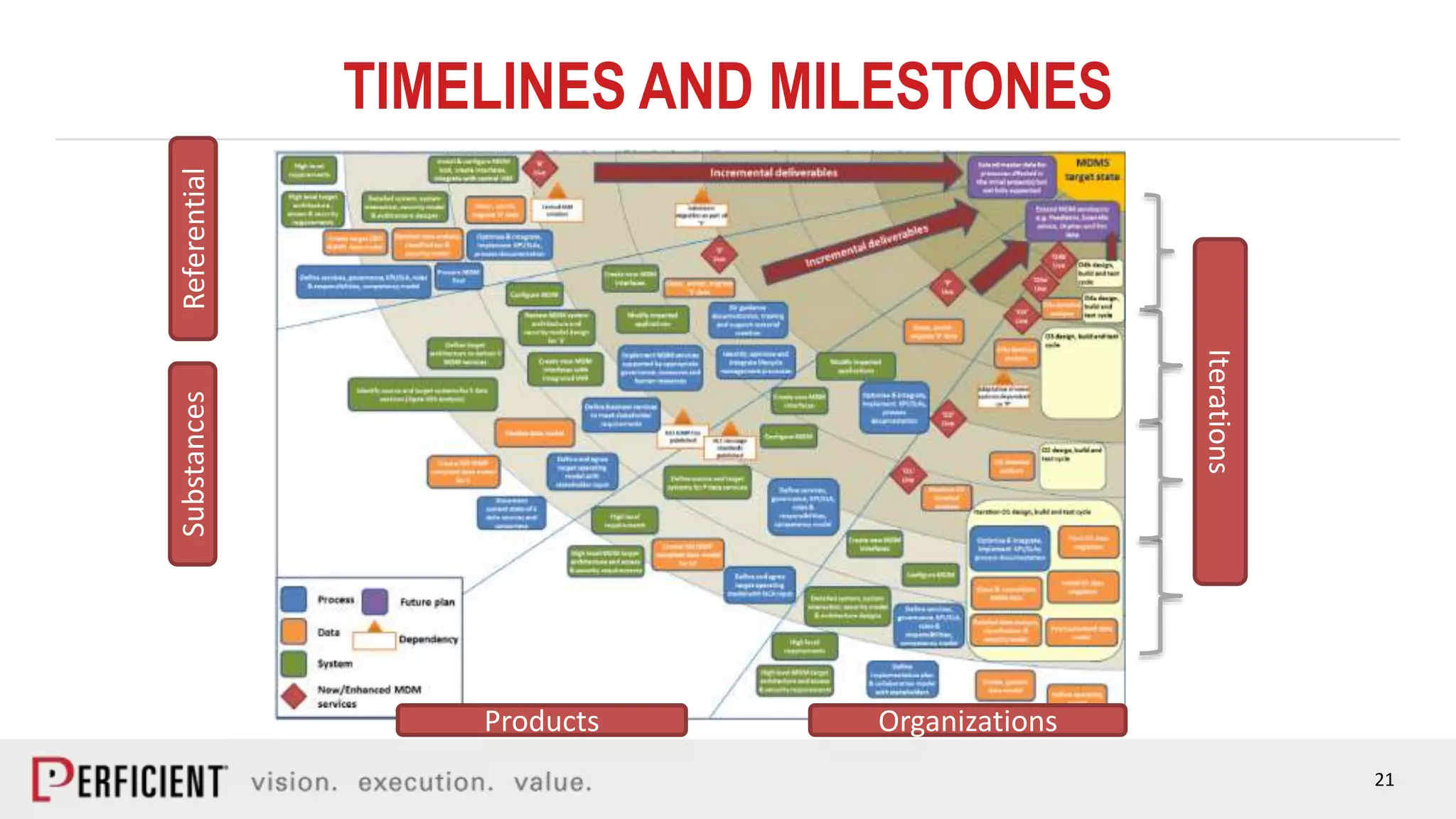
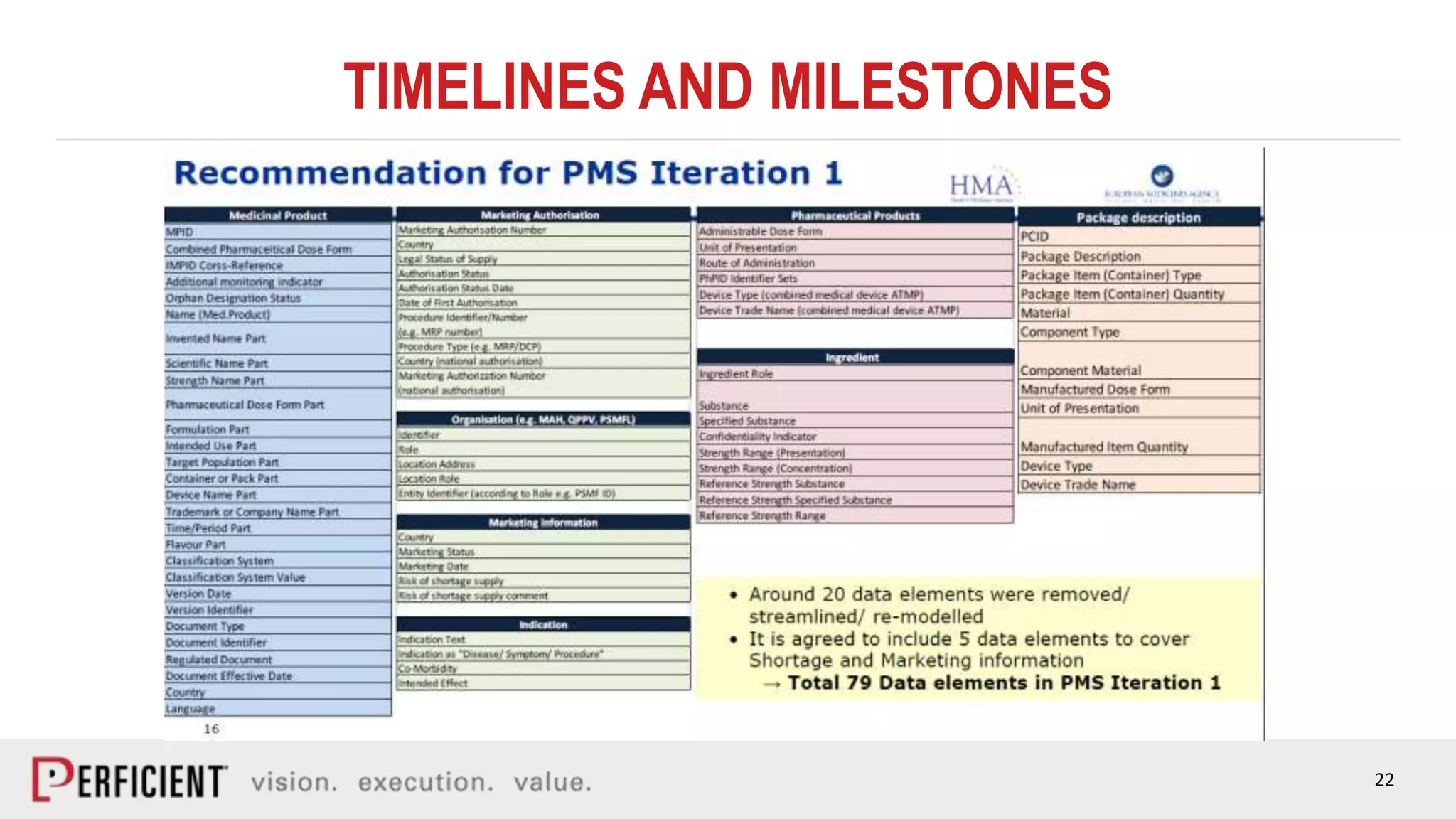
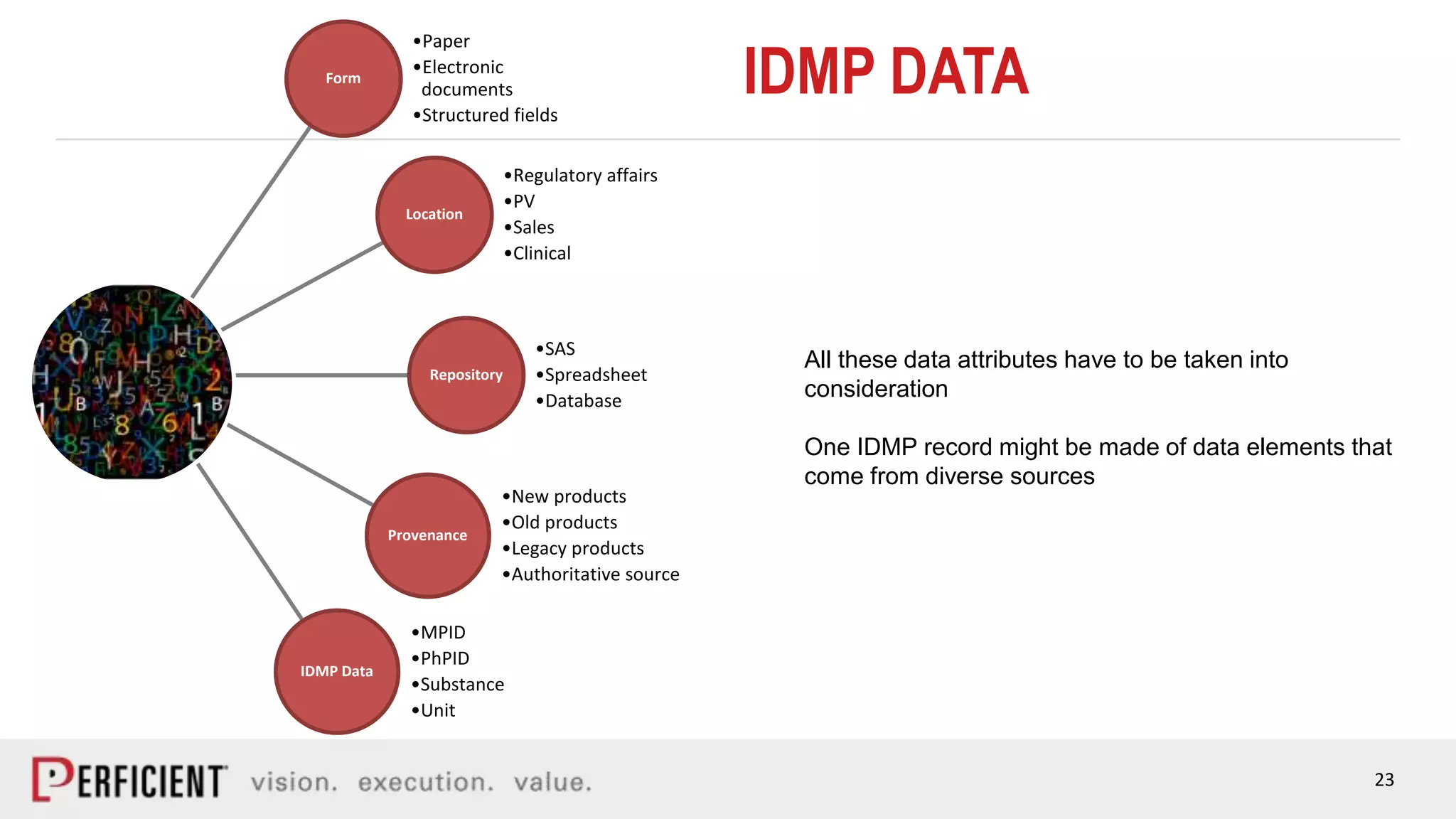
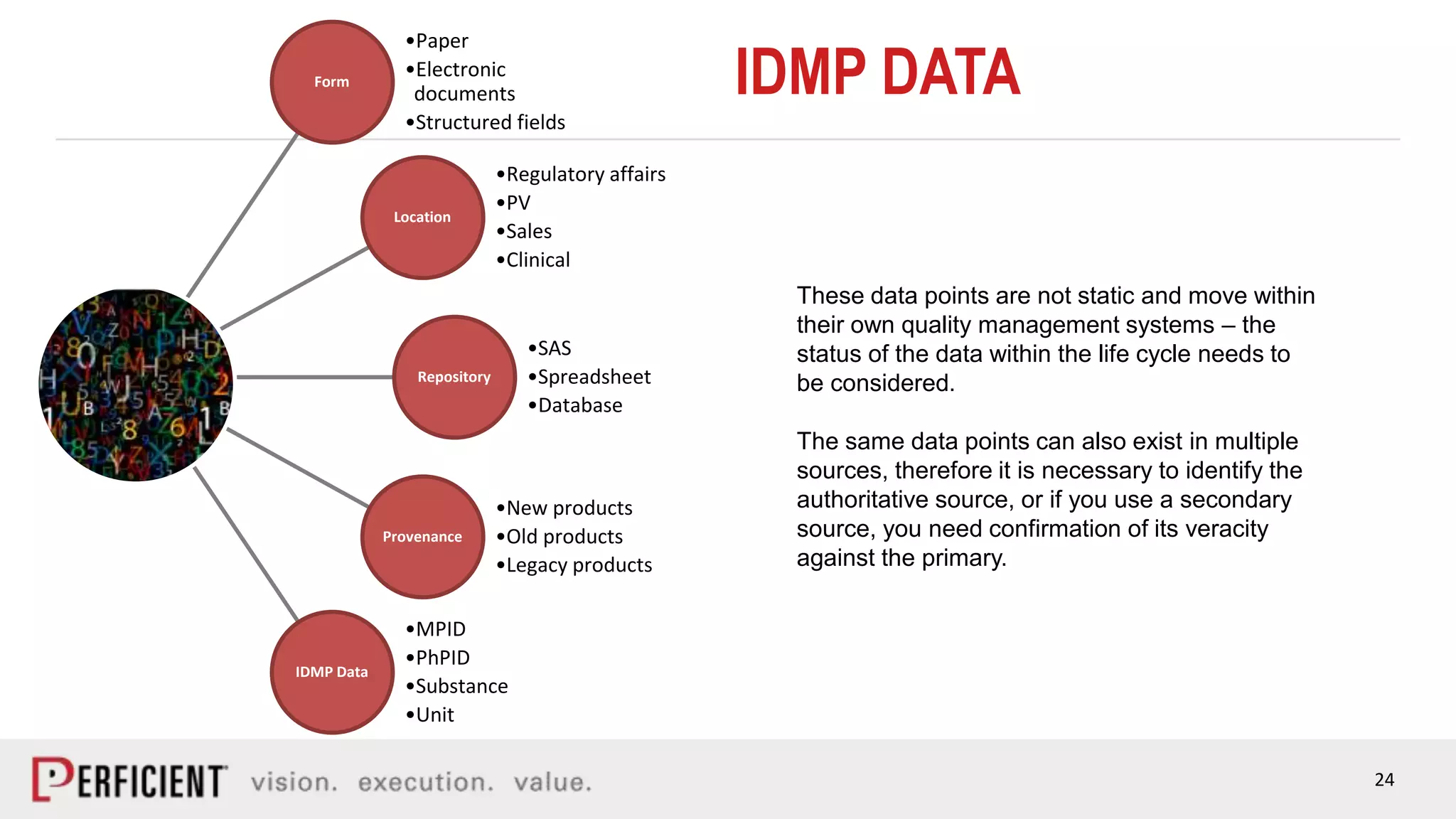
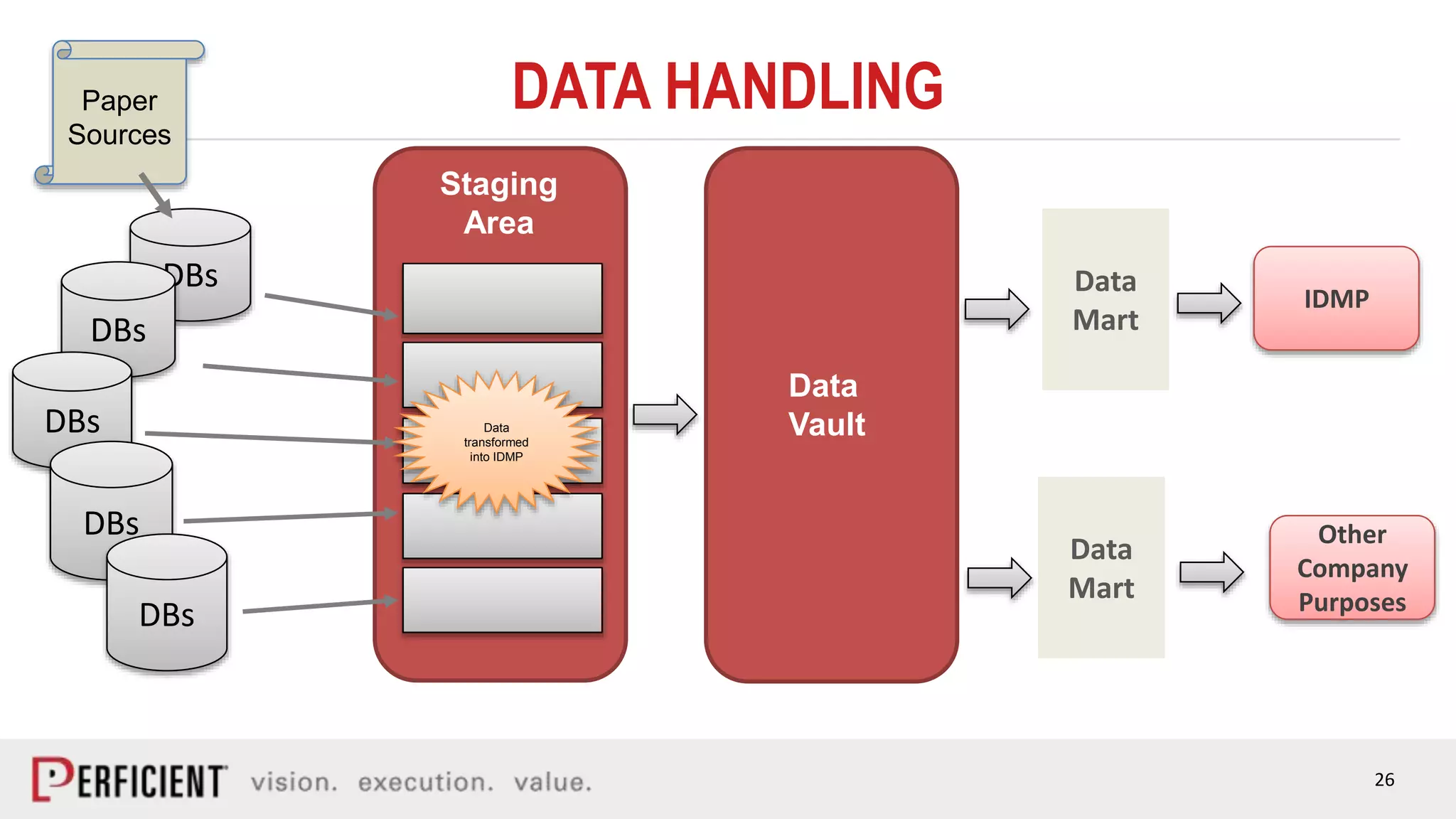
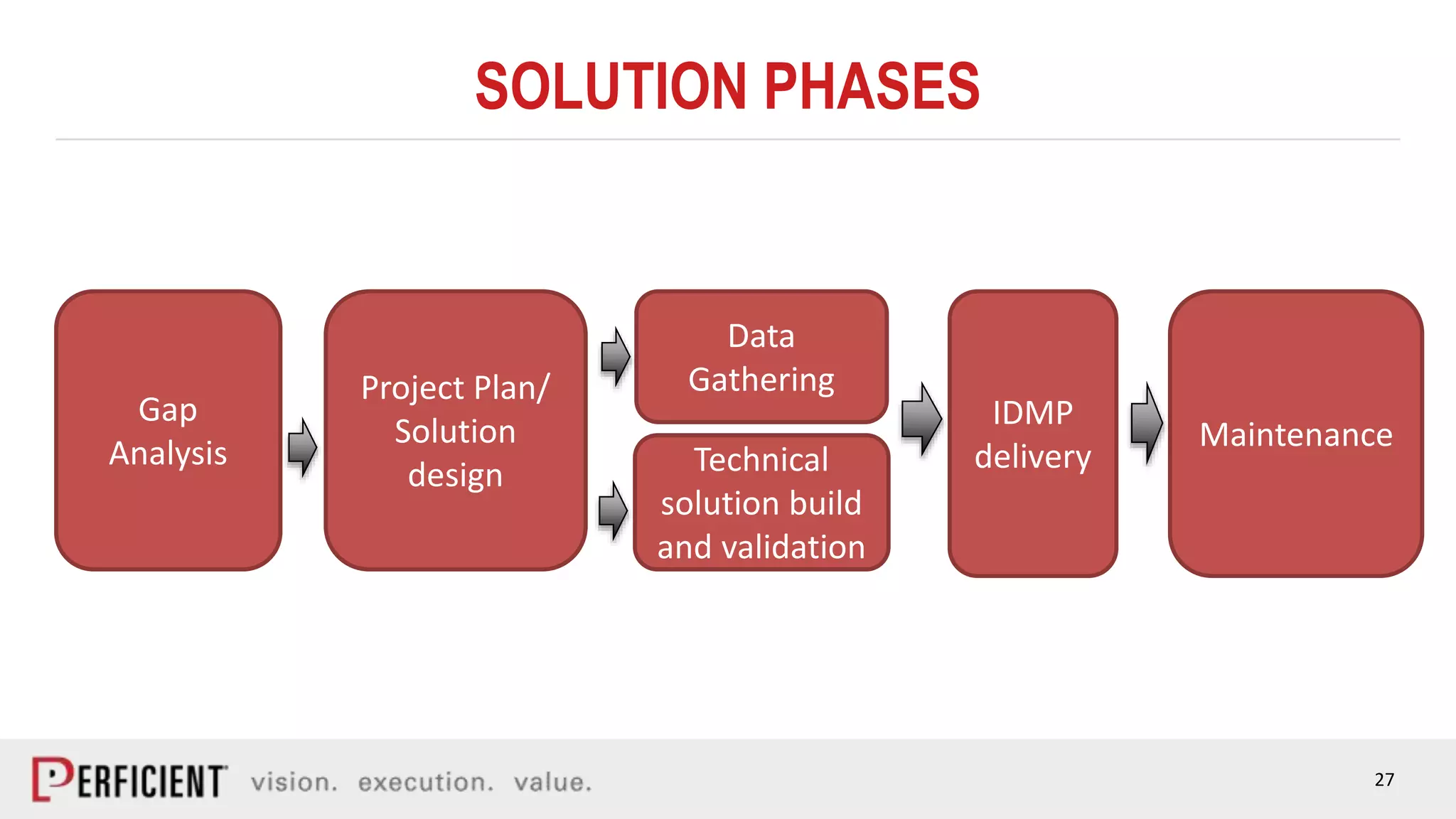
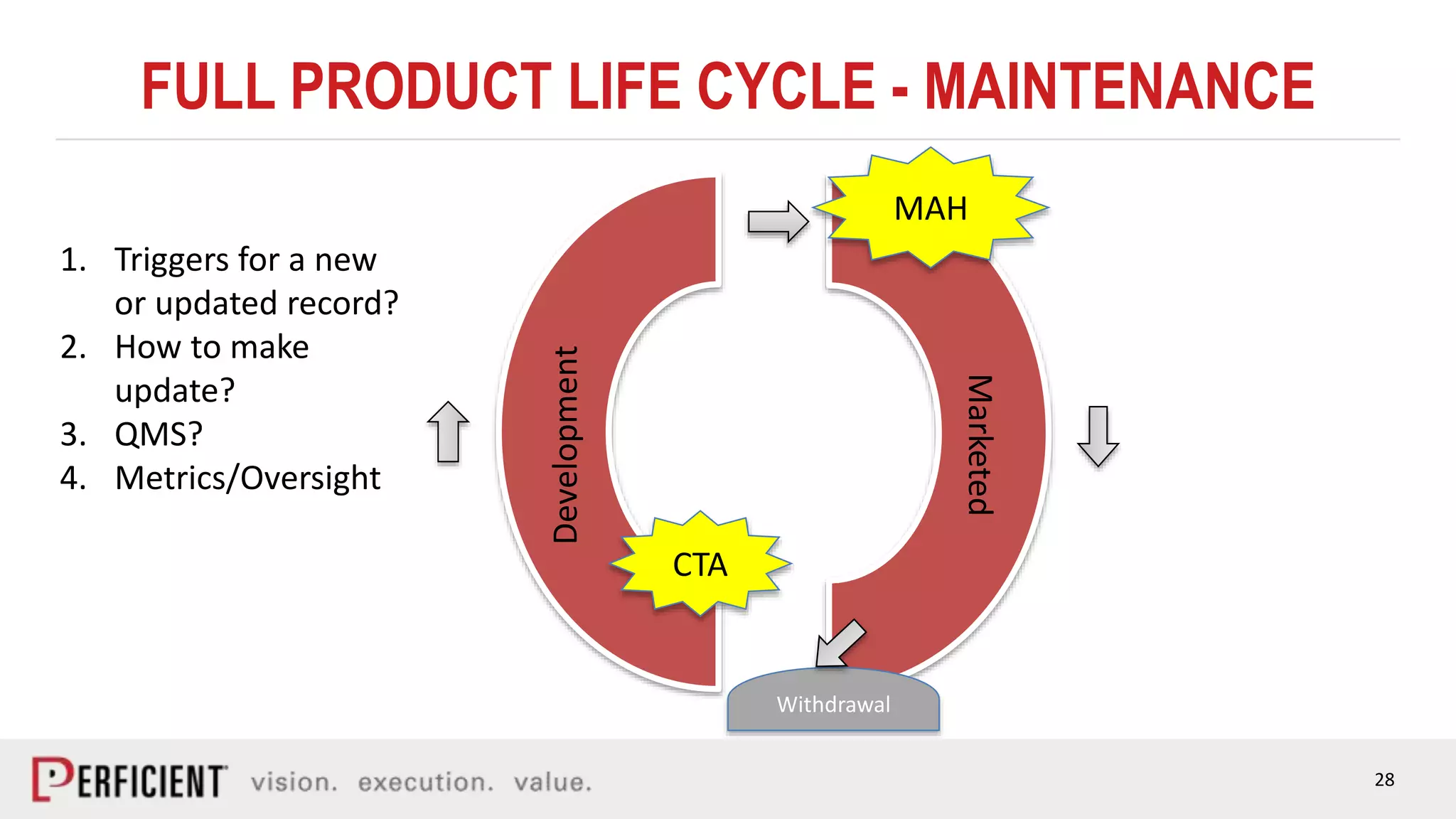
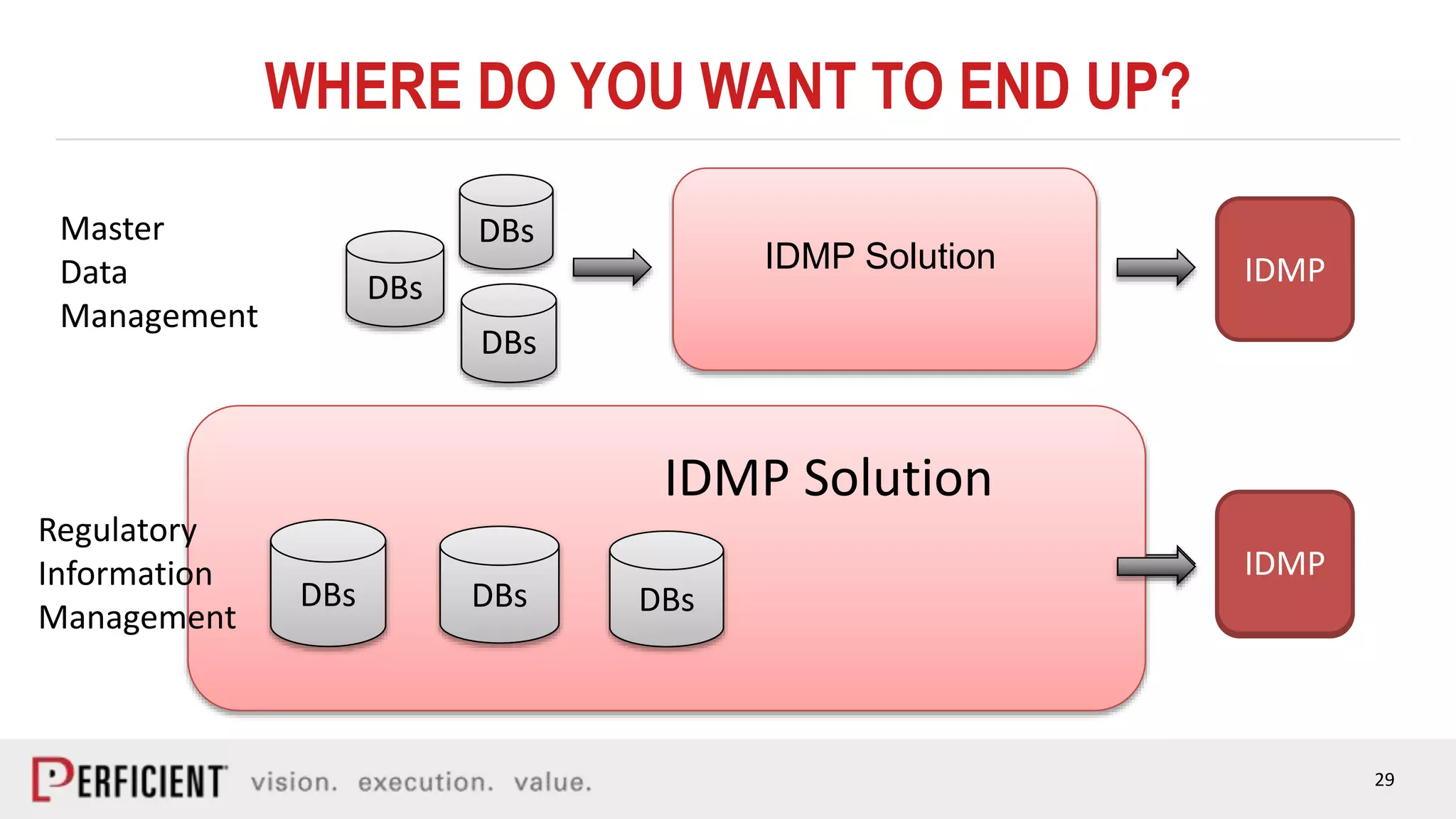
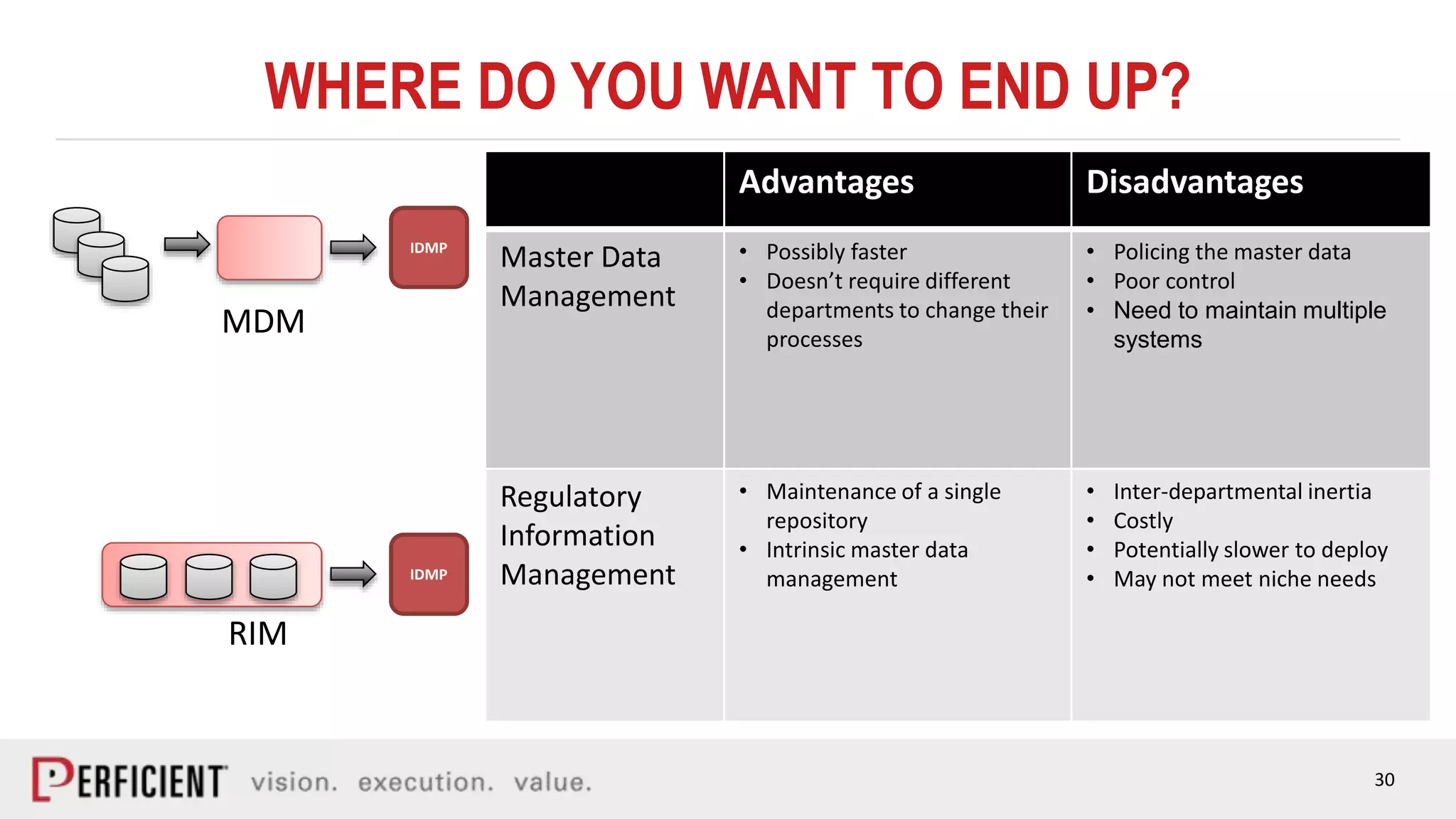
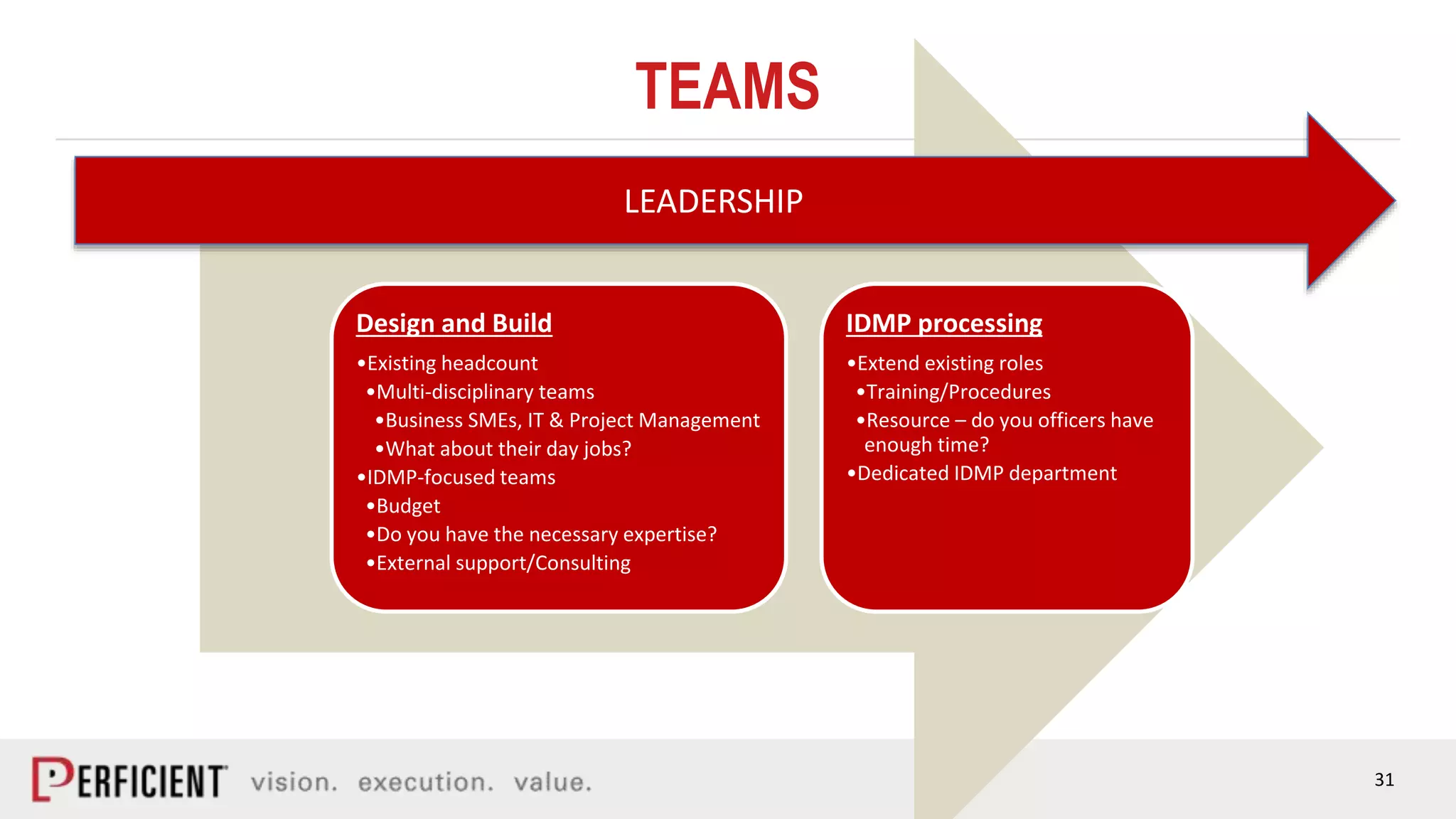
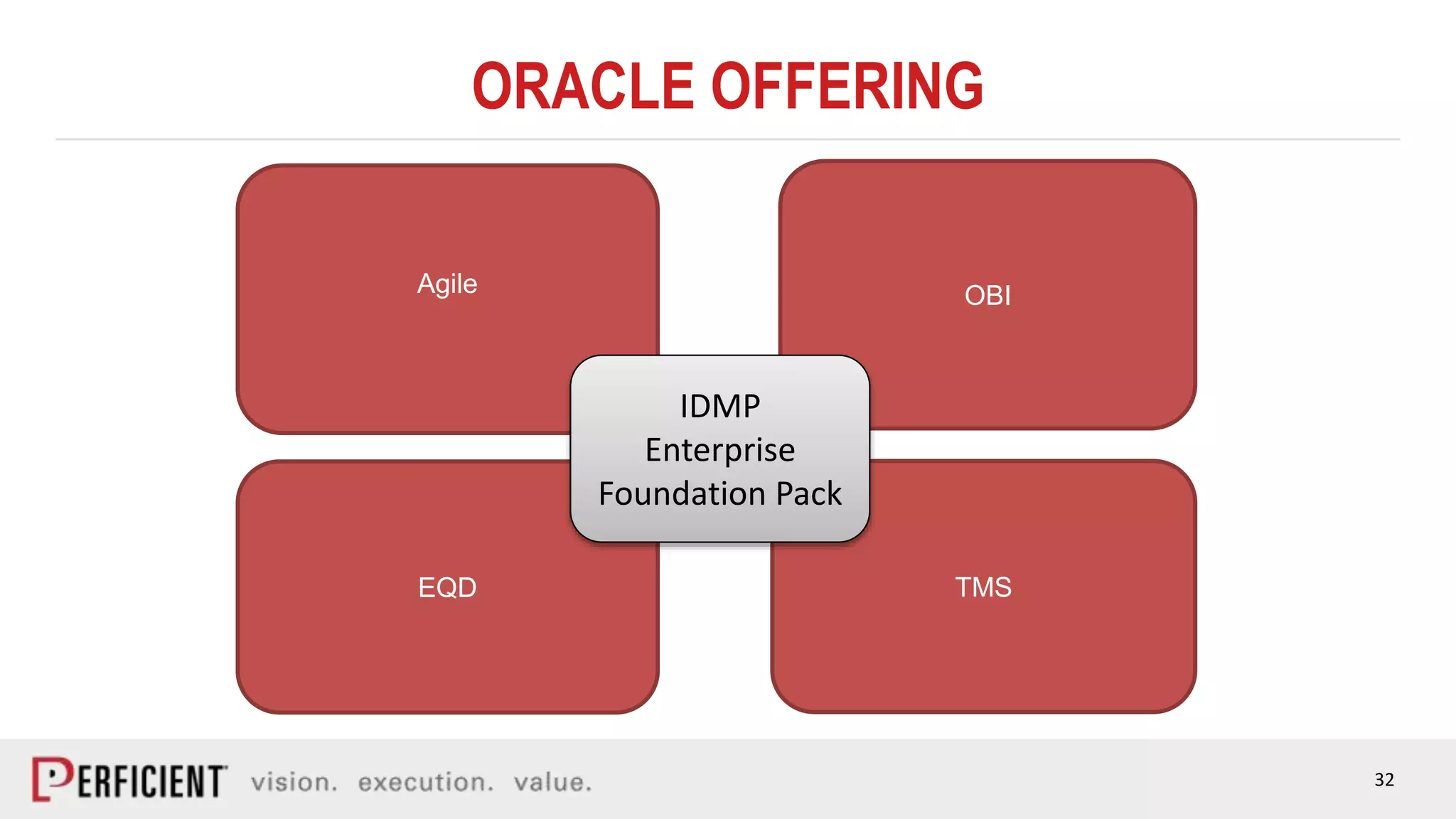
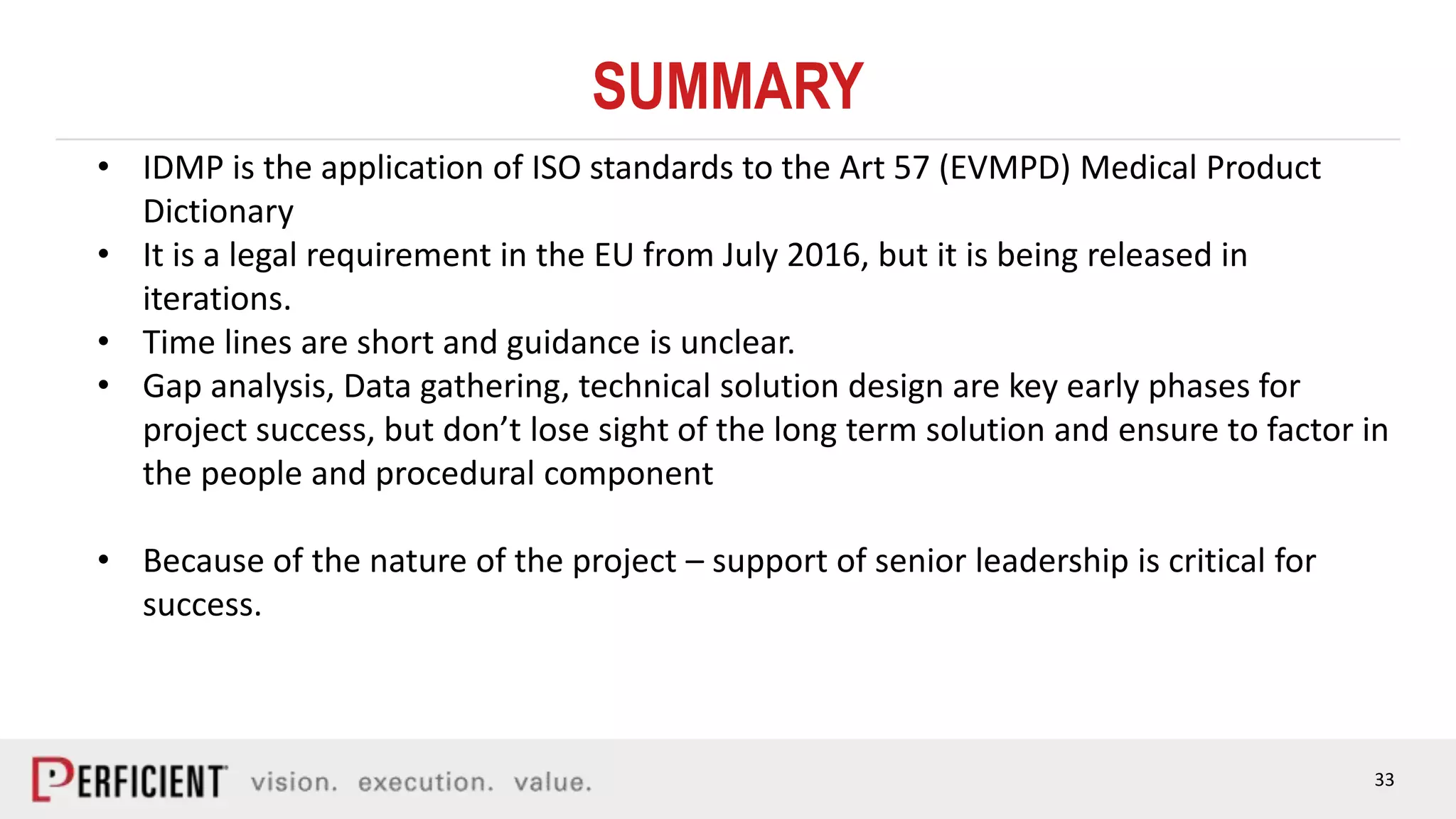
The document discusses the IDMP regulatory requirements and the role of Perficient as a consulting firm in helping organizations comply with these standards. It outlines the history and goals of IDMP, the relevant ISO standards, and the challenges of implementing these regulations in the pharmaceutical industry. Key phases for project success include gap analysis, data gathering, and technical solution design, emphasizing the need for senior leadership support.