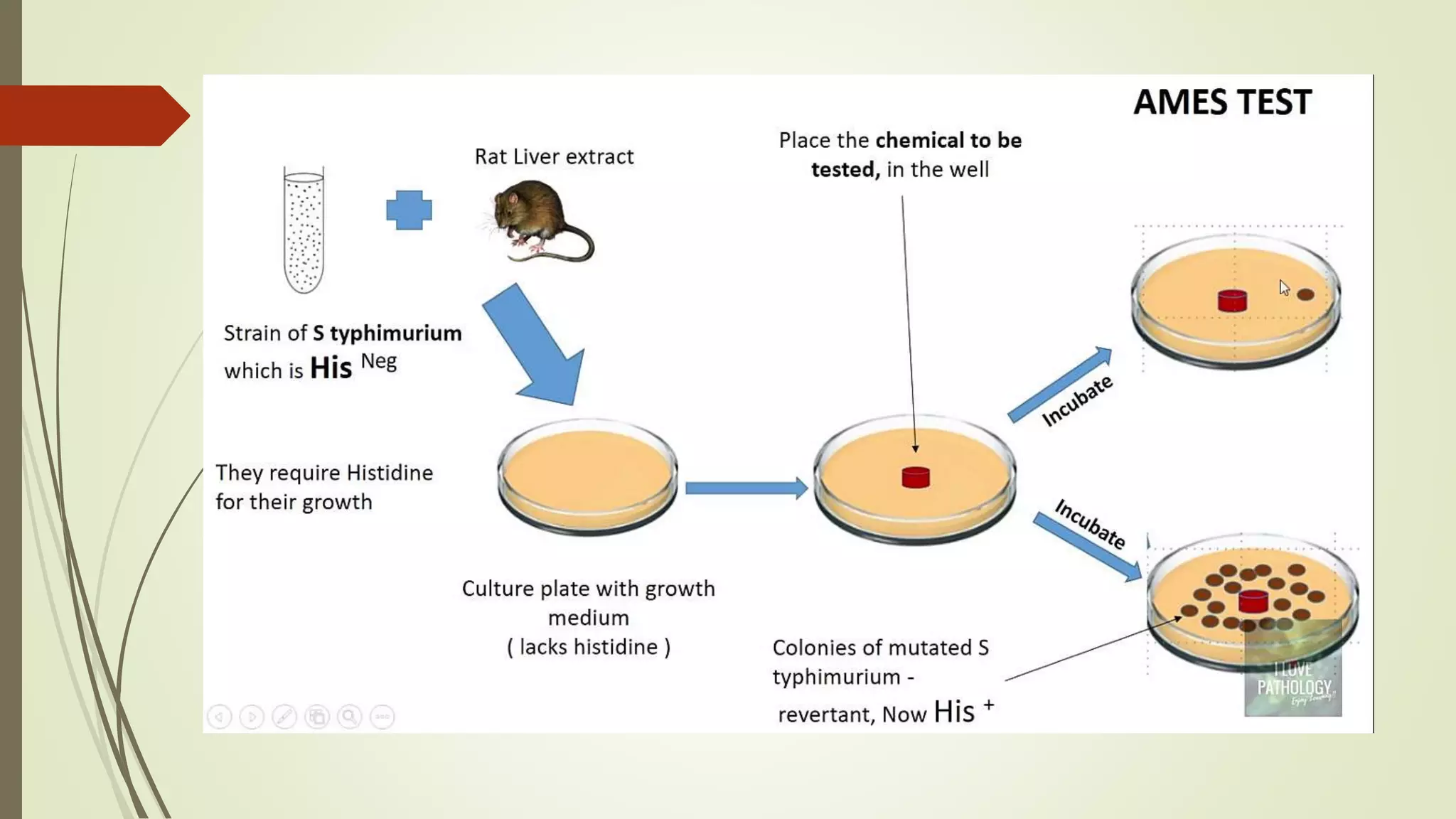
The Ames test uses mutant strains of Salmonella typhimurium bacteria to detect mutagenic and potentially carcinogenic chemicals. Bruce Ames developed the test in the 1970s as a less expensive alternative to animal testing. The test involves exposing bacteria to a test chemical and measuring any increase in mutations, as indicated by increased bacterial growth. If a chemical causes more bacterial growth compared to a control, it is considered mutagenic. The Ames test is widely used for screening chemicals and identifying potential carcinogens.