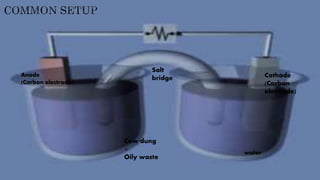
This document discusses how microorganisms can break down hydrocarbons in crude oil and oily waste. Certain bacteria like Alcanivorax, Marinobacter, Pseudomonas, and Acinetobacter are effective at degrading oil. These bacteria can break down organic pollutants from oil to produce electricity, hydrogen, and chemicals like caustic soda using a microbial electrolysis cell. Cow dung also shows potential for biodegrading petroleum products, with Bacillus, Pseudomonas, and Proteus species involved. Maximum degradation occurs at 37°C, pH 7 using xylene. The document then describes how a microbial electrolysis cell works to produce electricity from bacteria breaking down organic materials in oily waste