Today's science lesson will cover:
1. Physical vs chemical changes through notes and worksheets
2. Identifying volume through a lab
3. Test #2 on learning points from Oct 20-21 including notes and binder check
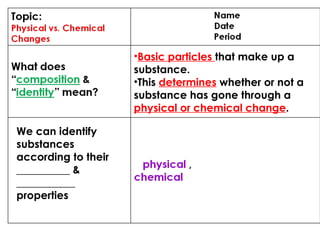
4. Basic particles that make up substances and how this determines if a change is physical or chemical
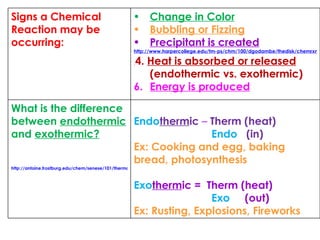
5. Signs that a chemical reaction may be occurring and examples of physical and chemical properties
6. The difference between endothermic and exothermic reactions