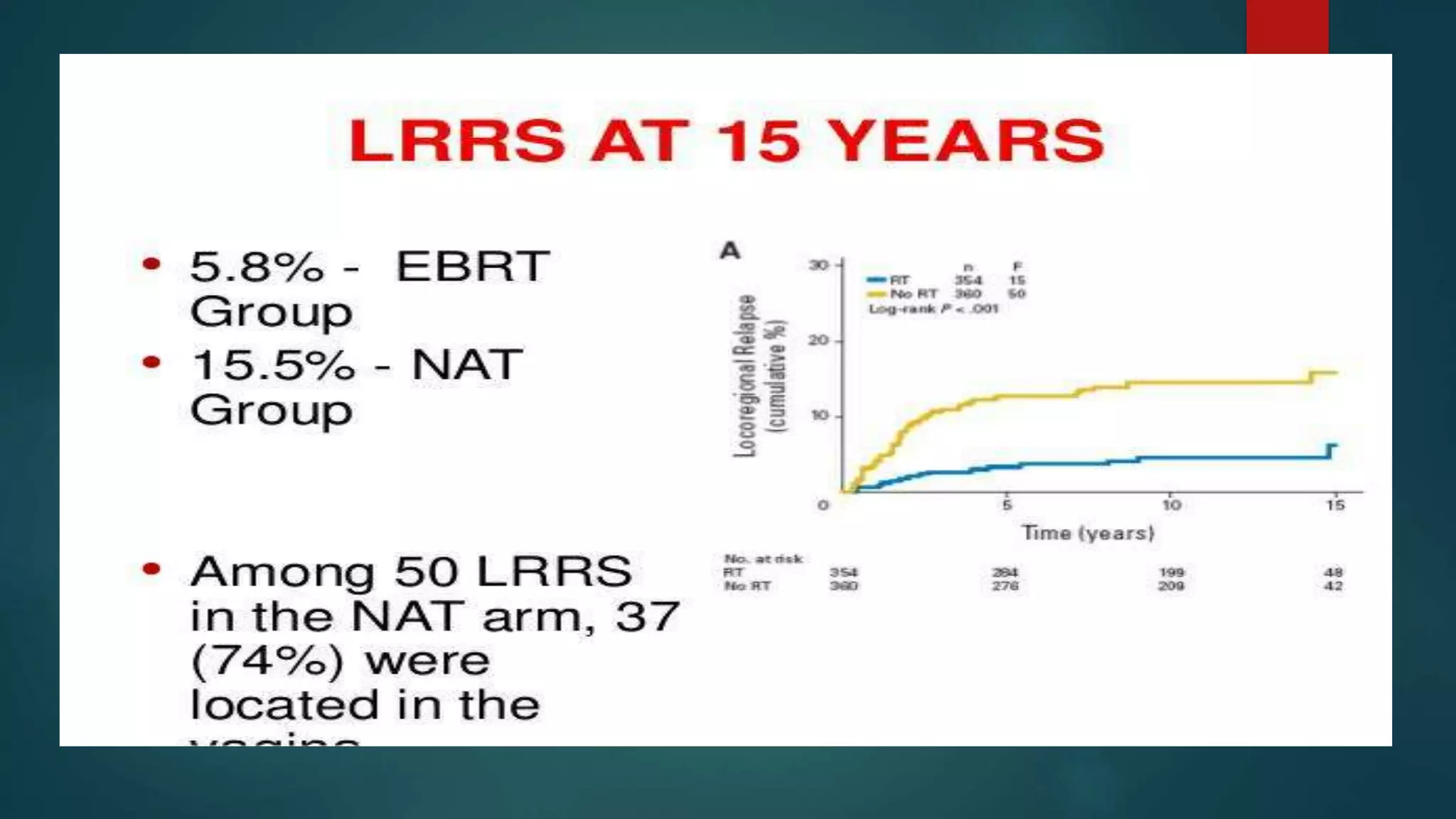
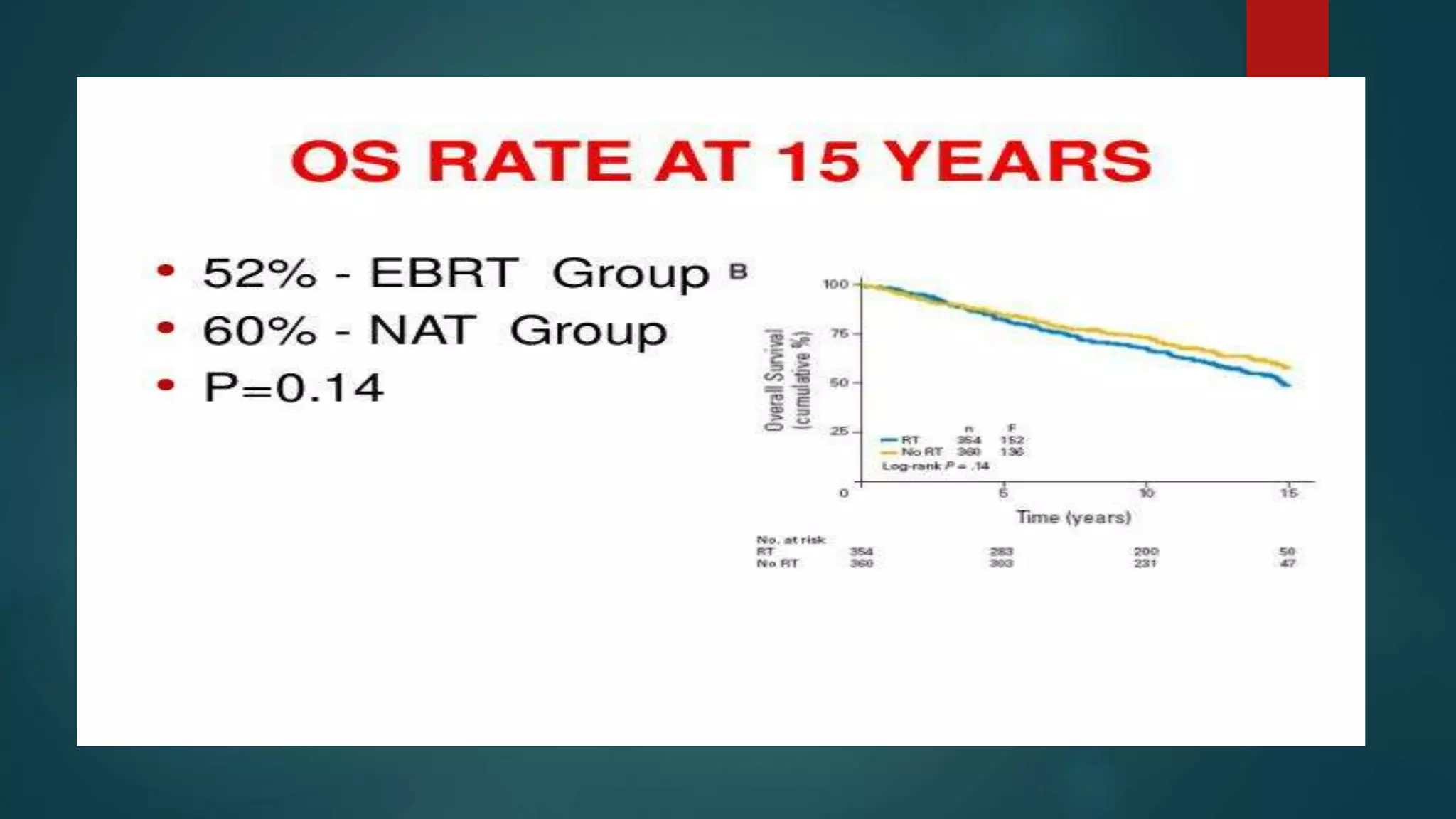
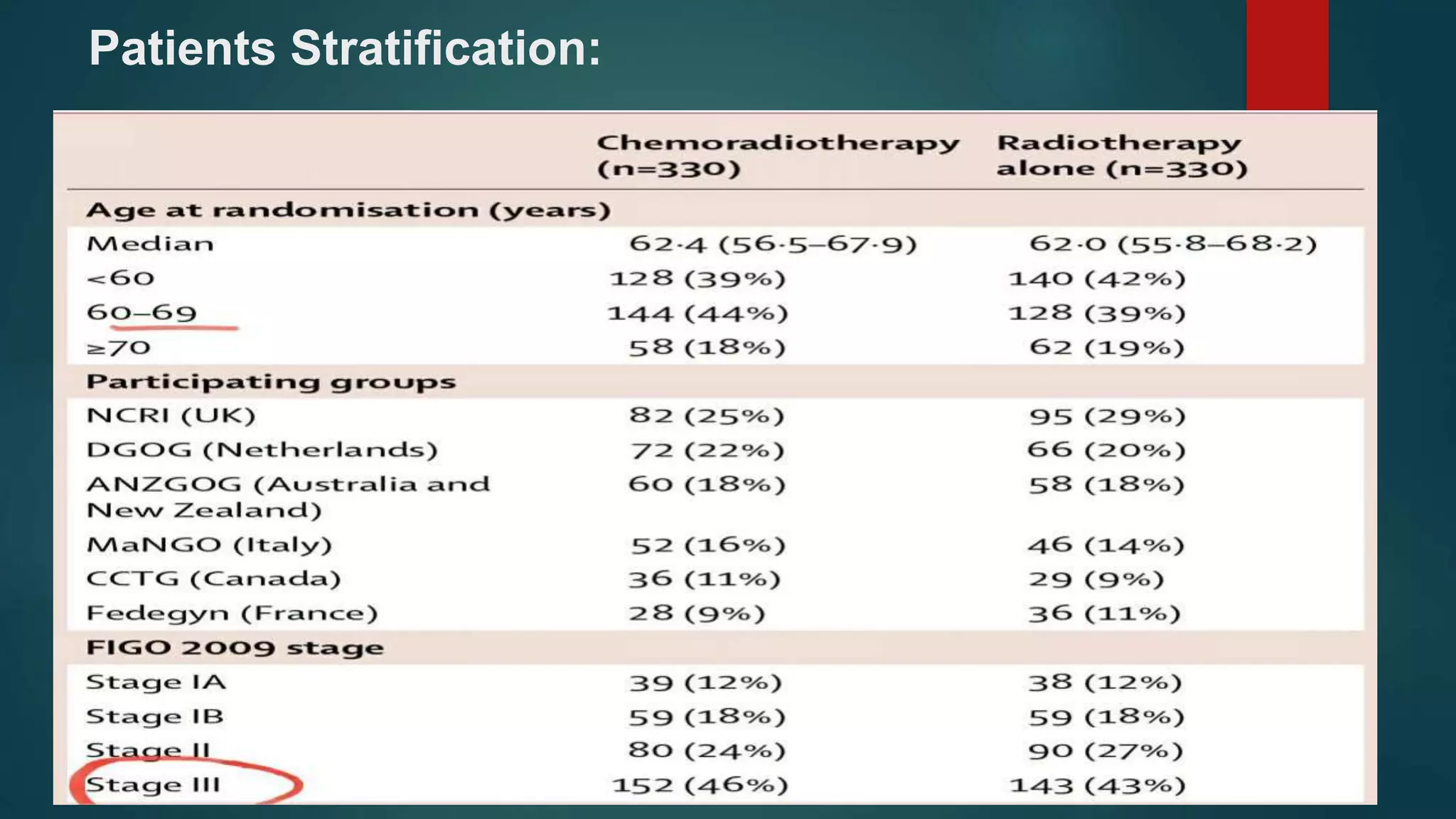
This document summarizes the results of the PORTEC-3 trial which compared adjuvant chemoradiation therapy (CTRT) to radiation alone in patients with high risk endometrial cancer. The trial included patients with high risk factors like grade 3 endometrioid cancer, stage II-III disease, or clear cell or serous histology. It found that while CTRT improved failure-free survival by 11% compared to radiation alone, there was only a 5% improvement in overall survival. CTRT was also associated with more toxicities but they were generally rapid to recover from. The benefits of CTRT need to be weighed against the increased costs and treatment duration associated with its toxicities. The conclusion