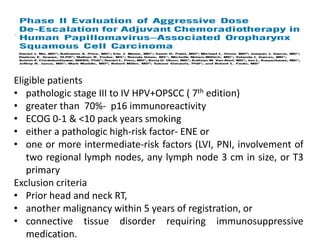
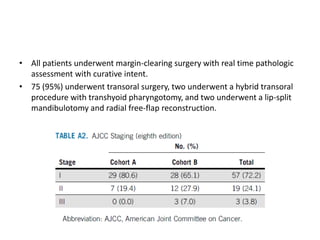
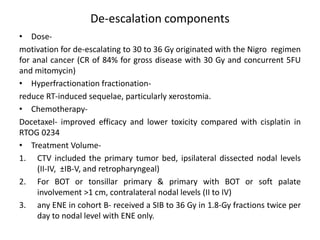
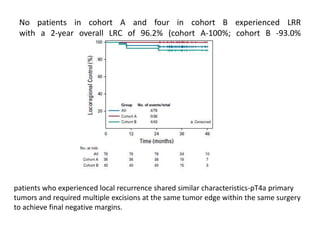
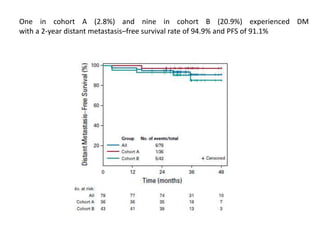
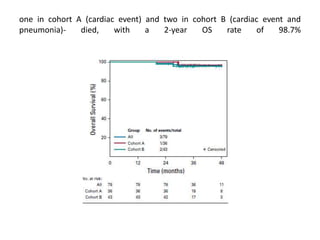
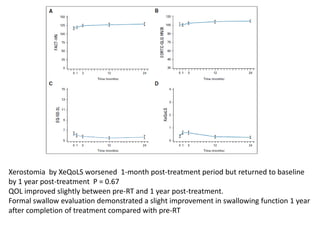
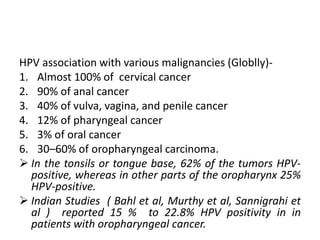
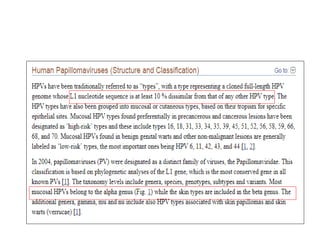
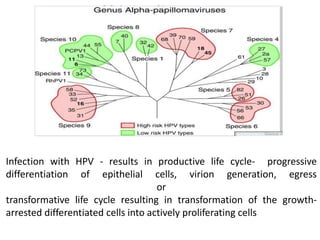
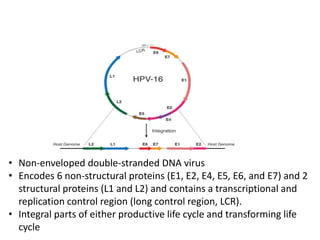
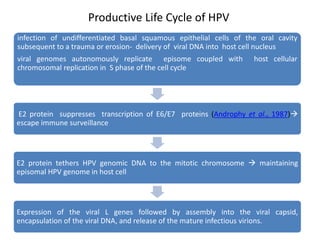
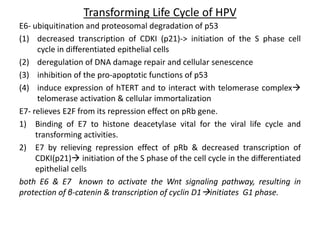
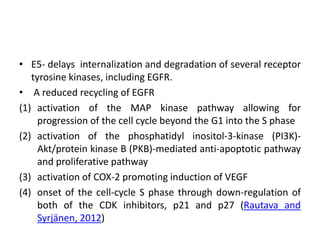
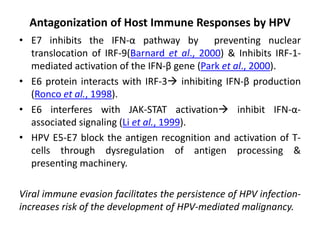
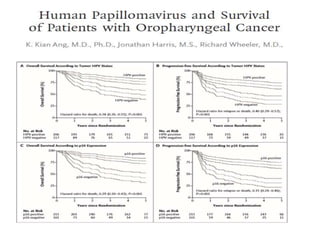
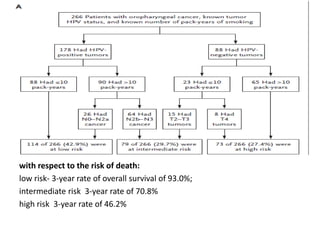
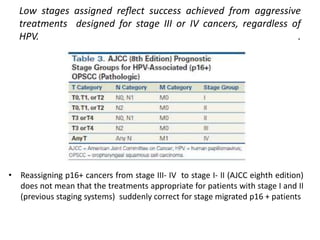
This document discusses treatment de-escalation strategies for HPV-positive oropharyngeal cancer. It provides details on the natural history of HPV and its life cycles. It also summarizes several clinical trials that aimed to de-escalate treatment intensity through strategies like reduced radiation doses, substituting chemotherapy agents, and limiting treatment volumes. One study found that substituting cetuximab for cisplatin reduced survival rates. Another trial found that induction chemotherapy followed by reduced radiation if patients responded well was feasible but came with increased toxicity. A third study found that transoral surgery followed by hyperfractionated radiotherapy with docetaxel achieved high rates of local control and survival with acceptable toxicity levels.



![De-ESCALaTE HPV
• 334 patients with low-risk
• The authors considered the use of cisplatin as the standard of care
and hence substitution with cetuximab could be considered
deviation or de-escalation from the established standard.
• primary outcome- (Grade 3–5) toxicity events
• secondary outcomes- OS , time to recurrence, quality of life, and
swallowing outcomes
• significantly higher number of serious adverse events in the
cisplatin group.
• No difference in overall severe toxicity, QOL or Swallowing otcomes
between the two groups
Far more concerning was the significant reduction in 2-year overall
survival (97·5% vs 89·4%, hazard ratio [HR] 5·0 [95% CI 1·7–14·7],
p=0·0012) and increase in disease recurrence (6·0% vs 16·1%, HR 3·4
[1·6–7·2], p=0·0007) observed in the cetuximab group.](https://image.slidesharecdn.com/hpvopc1-190830043817/85/HPV-OPSCC-De-escalation-Strategies-16-320.jpg)