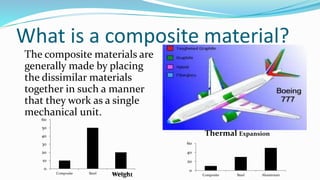
Composite materials are made by combining two or more dissimilar materials to take advantage of their combined properties. They consist of a matrix phase that binds together the dispersed phase to form a single mechanical unit. Composites offer advantages over traditional materials like metals and polymers, including higher strength and stiffness for a given weight, better corrosion and heat resistance, and easier fabrication. Common applications of composites include use in vehicles, sports equipment, electronics and construction.






![MANUFACTURE OF WHITE POTTERY
Step I Preparation of
body ware
The raw materials Kaoline
[Al (OH)4 SiO5] and
Feldspar are made into fine
powder and mixed with
water to form a creamIt is
dried and then fired in a
'biscuit' oven to get porous
ware called 'Bisque’](https://image.slidesharecdn.com/unit-iv-150313110101-conversion-gate01/85/Poly-chemIV-9-320.jpg)