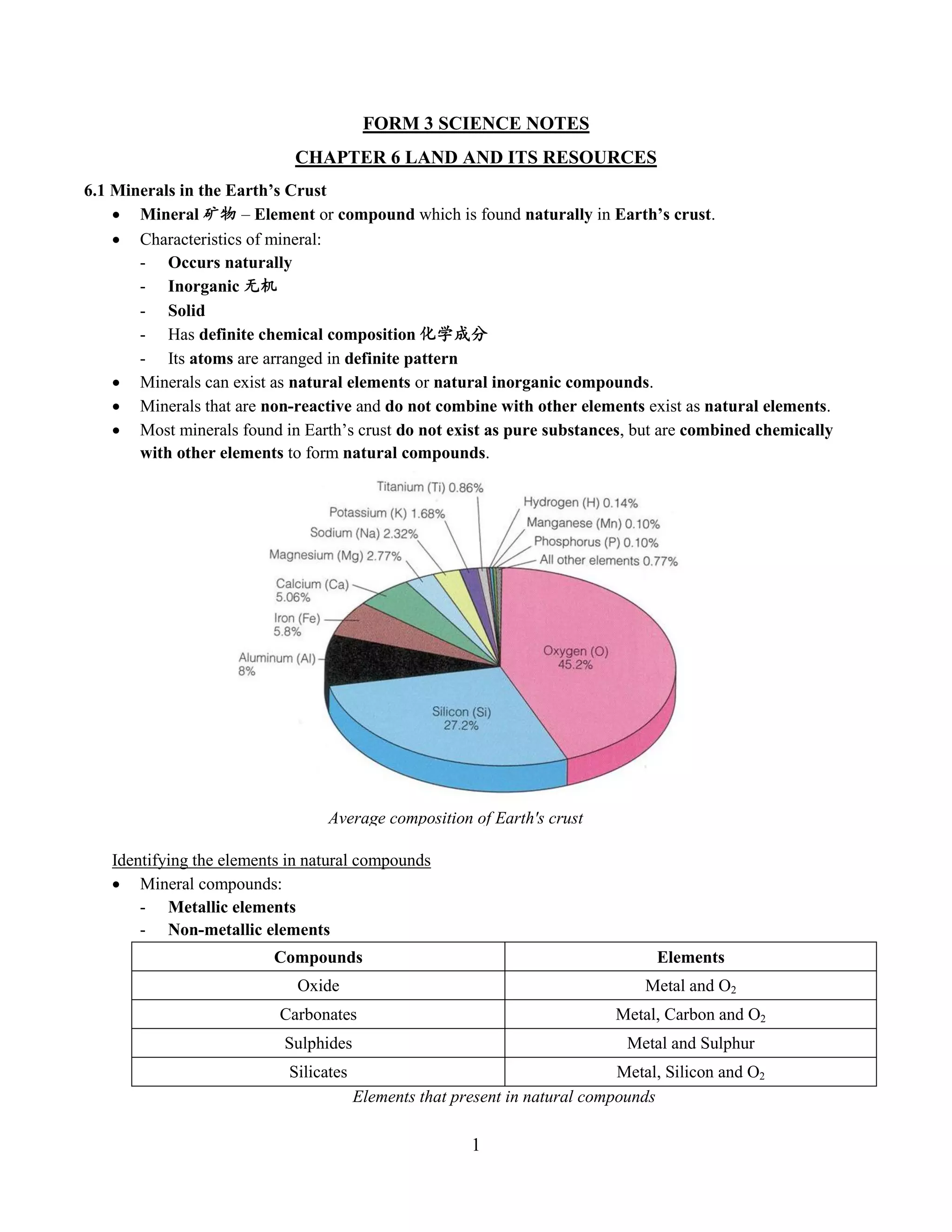
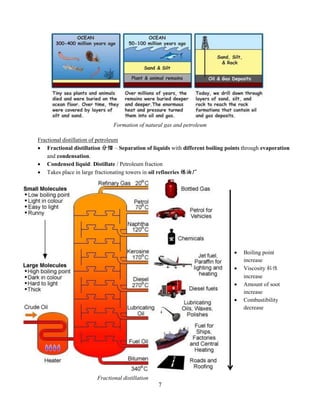
This document summarizes key information about minerals, metals, and natural fuel resources found in Earth's crust. It discusses the characteristics of minerals like their chemical composition and properties. Common minerals are described along with the chemical reactions of metals and non-metals. Natural fuel resources like coal, petroleum, and natural gas are formed from decayed organic matter and are important to Malaysia's economy. Efficient use of these resources through practices like energy conservation and recycling can help reduce consumption.