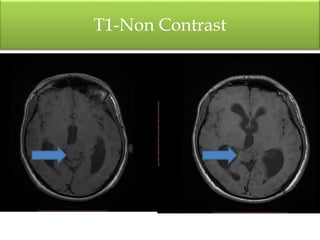
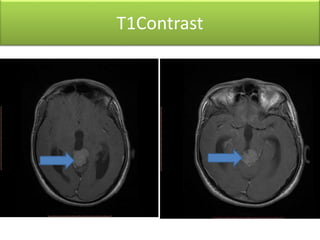
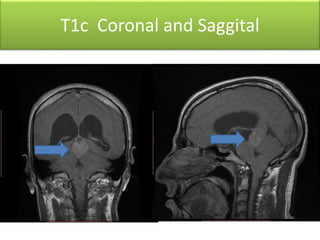
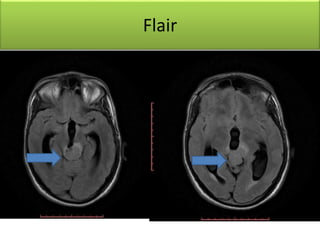
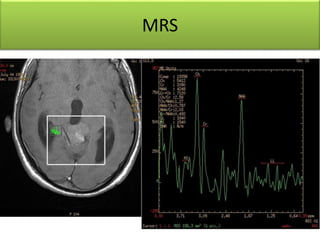
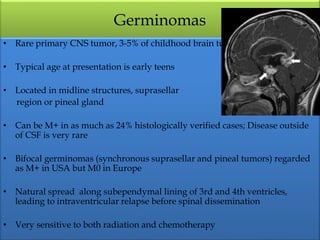
1) Pineoblastoma and germ cell tumors are rare intracranial tumors, with pineoblastoma occurring most often in young children. Complete surgical resection is difficult due to tumor location.
2) Treatment involves maximal safe surgical resection followed by chemotherapy and craniospinal irradiation. Younger children (<3 years) have a poorer prognosis and require more intensive treatment regimens.
3) Older children (>3 years) have shown improved survival when treated with chemotherapy and craniospinal irradiation after surgery. Younger children have generally not responded well to chemotherapy alone.