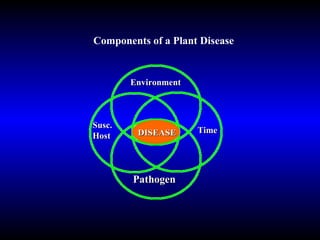
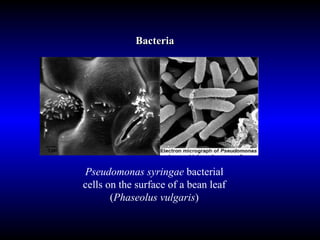
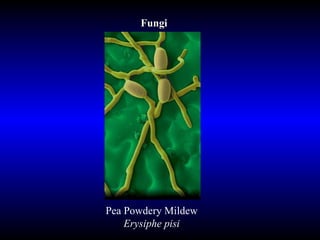
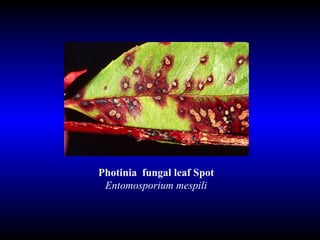
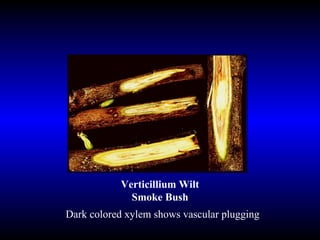
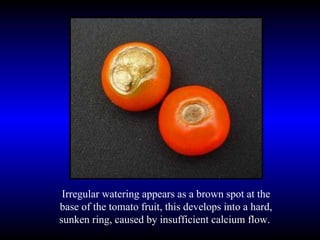
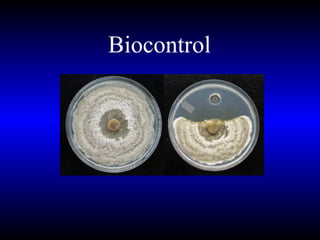
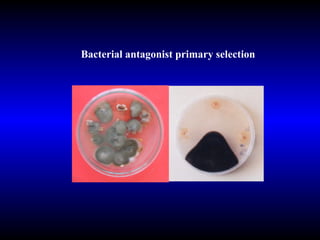
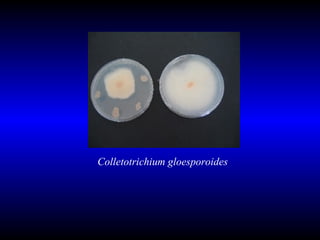
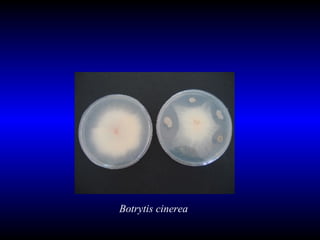
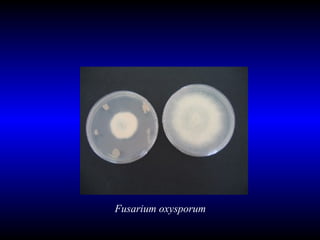
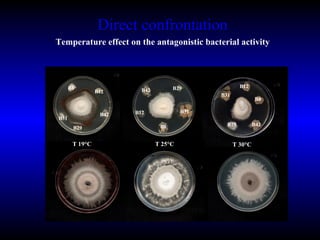
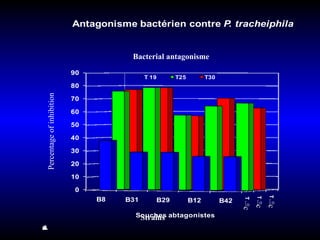
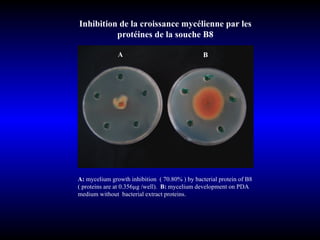
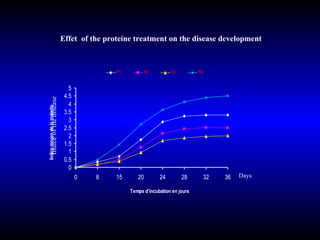
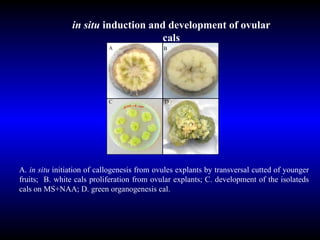
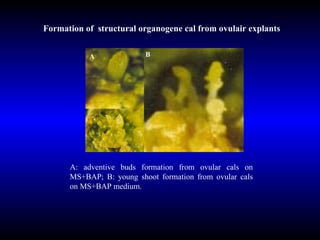
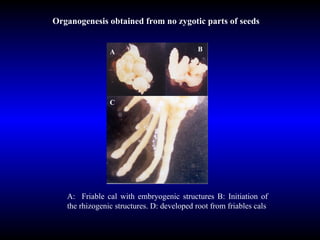
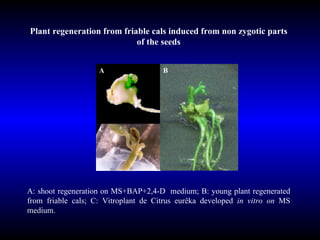
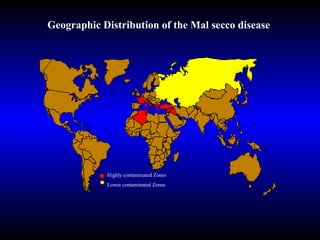
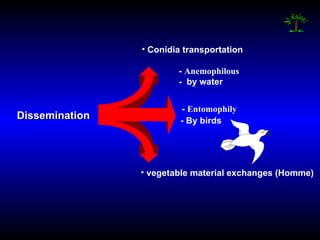
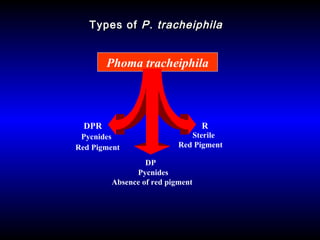
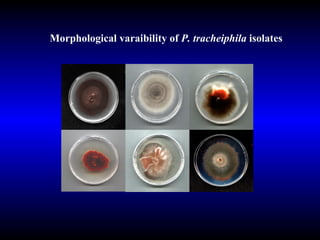
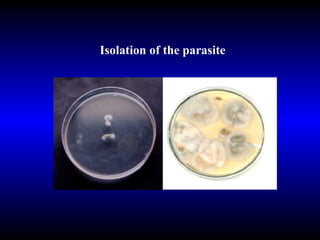
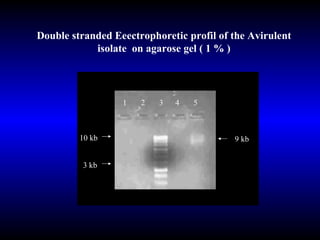
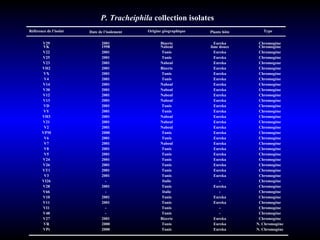
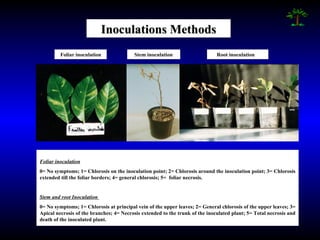
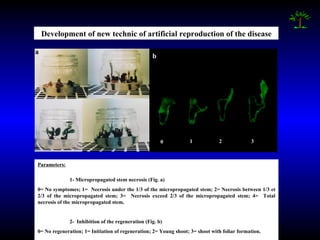
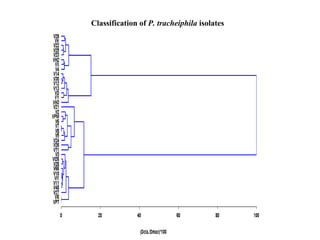
Phytopathology is the study of plant diseases caused by pathogens and physiological factors. The document discusses various plant pathogens like viruses, bacteria, fungi, nematodes and parasitic plants that cause different plant diseases. It also describes methods of disease control which include chemical treatments, biocontrol methods and developing resistant plant varieties through cultural practices and breeding.