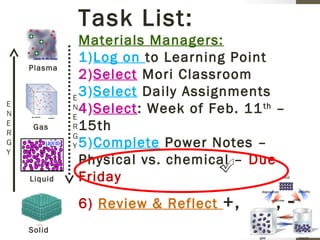
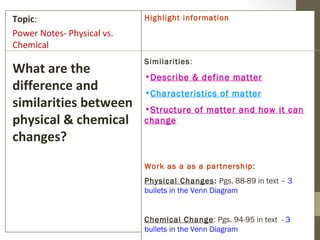
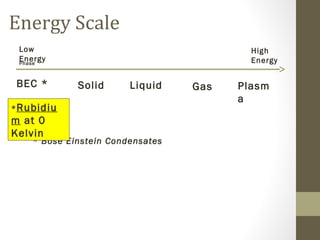
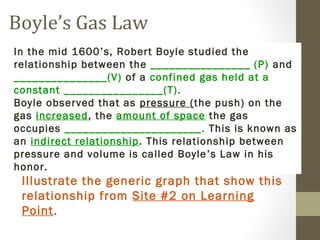
The document provides instructions and assignments for a science class, including completing power notes on physical versus chemical changes, reviewing gas laws illustrated with videos and graphs, and answering questions about the topics. Students are asked to log assignments, gather materials, finish tasks by certain dates, and turn in work individually or with group members. The document outlines the daily plan, assignments, and expectations for the science lesson.