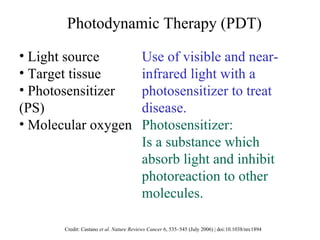
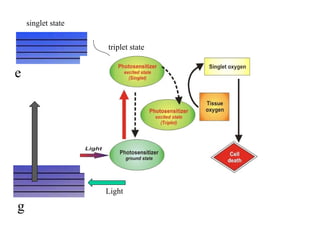
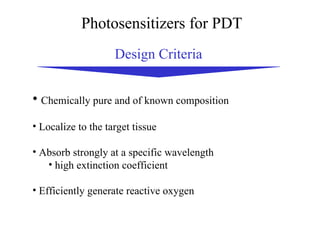
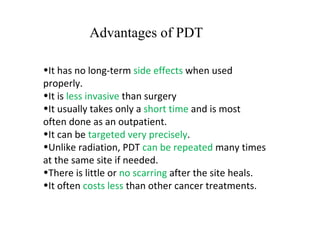
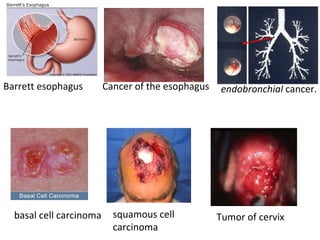
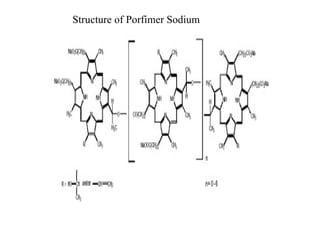
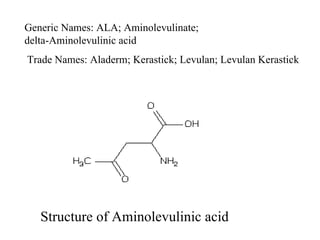
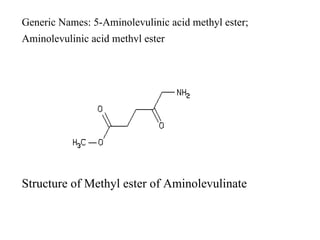
The document discusses photodynamic therapy (PDT), a treatment that uses photosensitizing drugs activated by light to treat cancer and other diseases. It describes several FDA-approved photosensitizing drugs including porfimer sodium, aminolevulinic acid, methyl ester of ALA, and verteporfin. The document also discusses the mechanism of PDT, advantages, potential side effects of the drugs, and future directions for improving PDT treatments.



















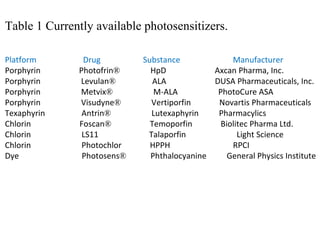



![They may be removed from the body more quickly, reducing the time people need to worry about photosensitivity reactions. An example of one of these new drugs, Photochlor®, is now being used in clinical trials. Photochlor or HPPH (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a) is a second-generation photosensitizer.](https://image.slidesharecdn.com/photodyanamictherapyppt-110527021004-phpapp01/85/Photodyanamic-therapy-ppt-38-320.jpg)


