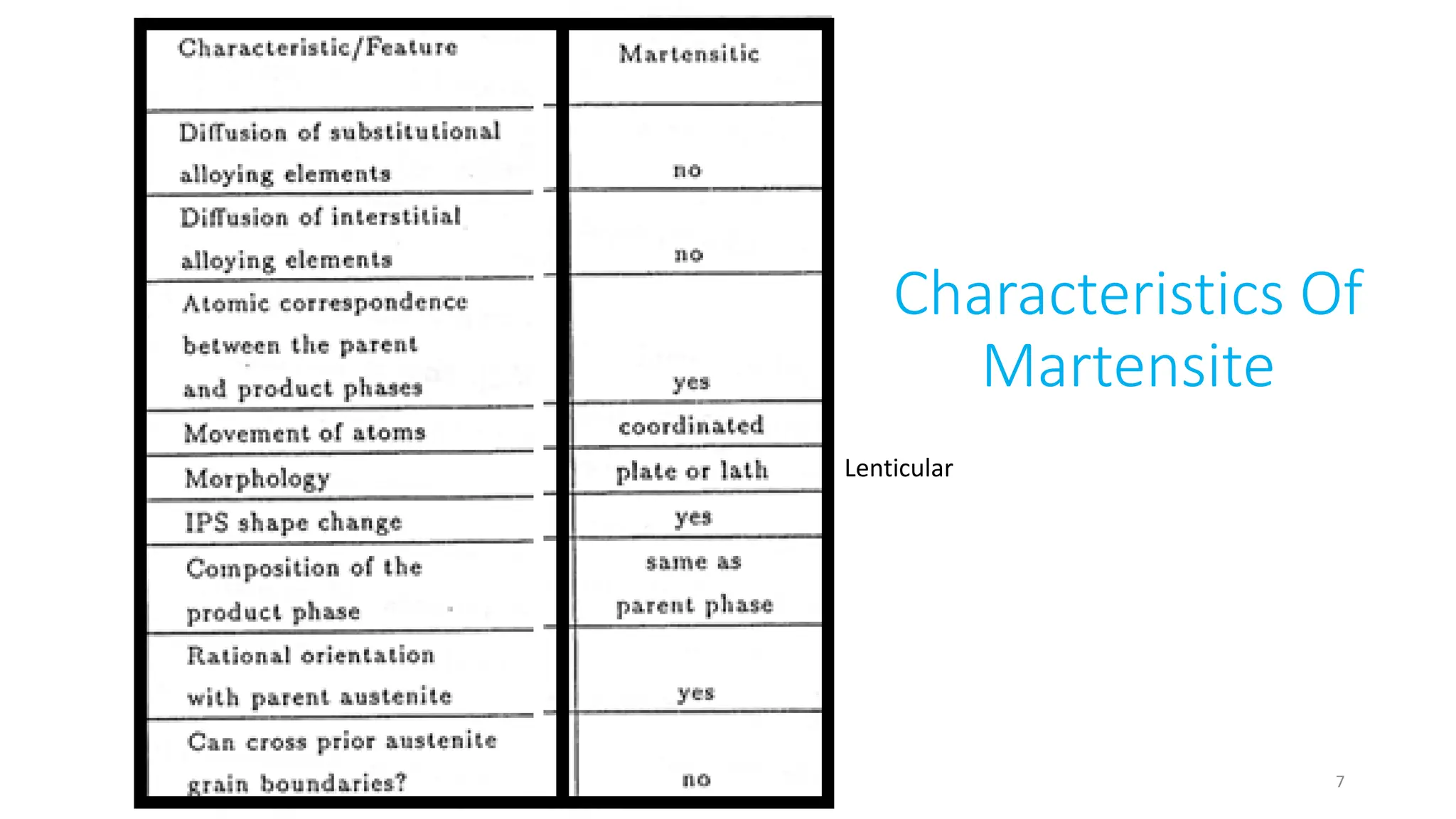
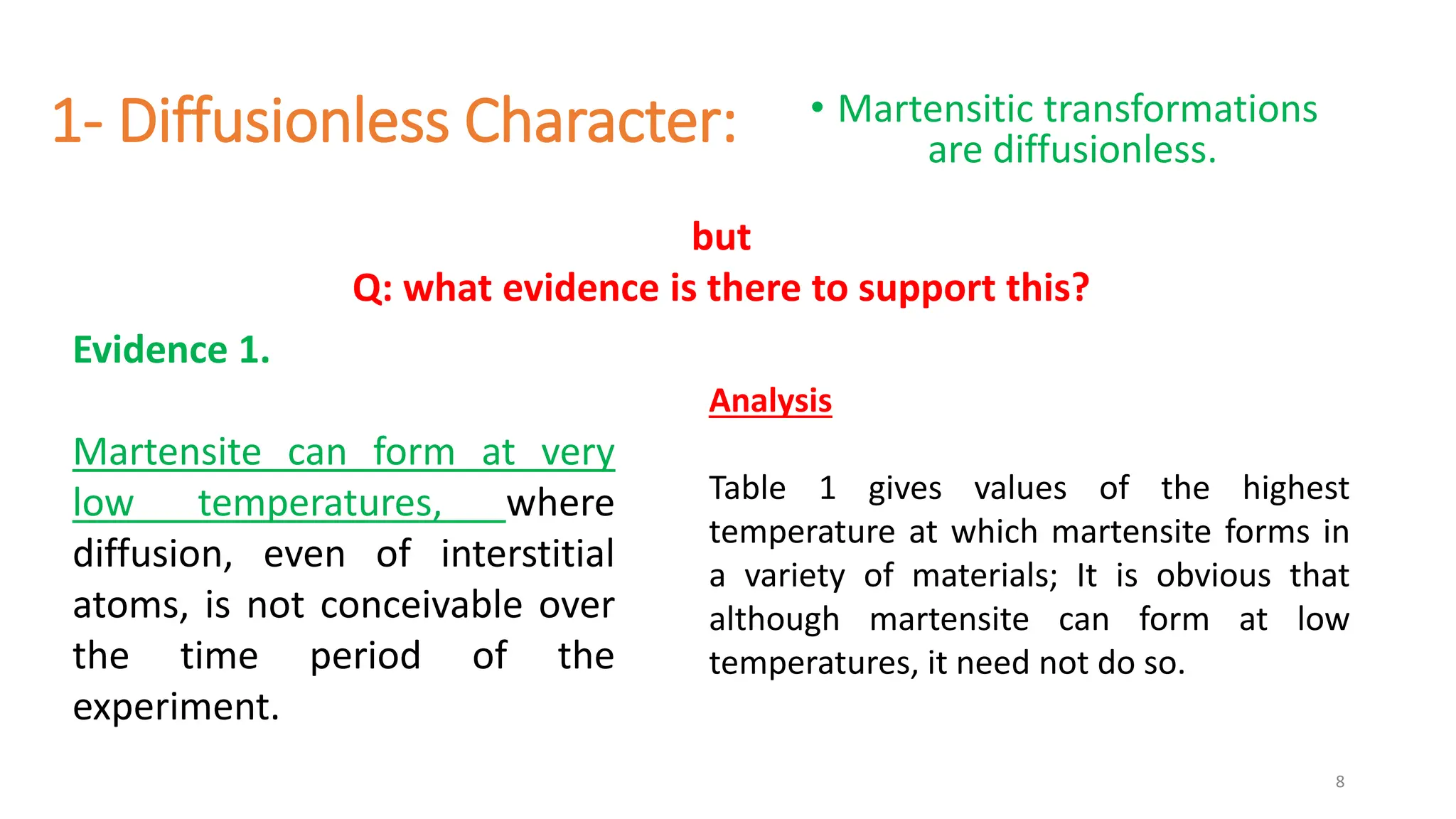
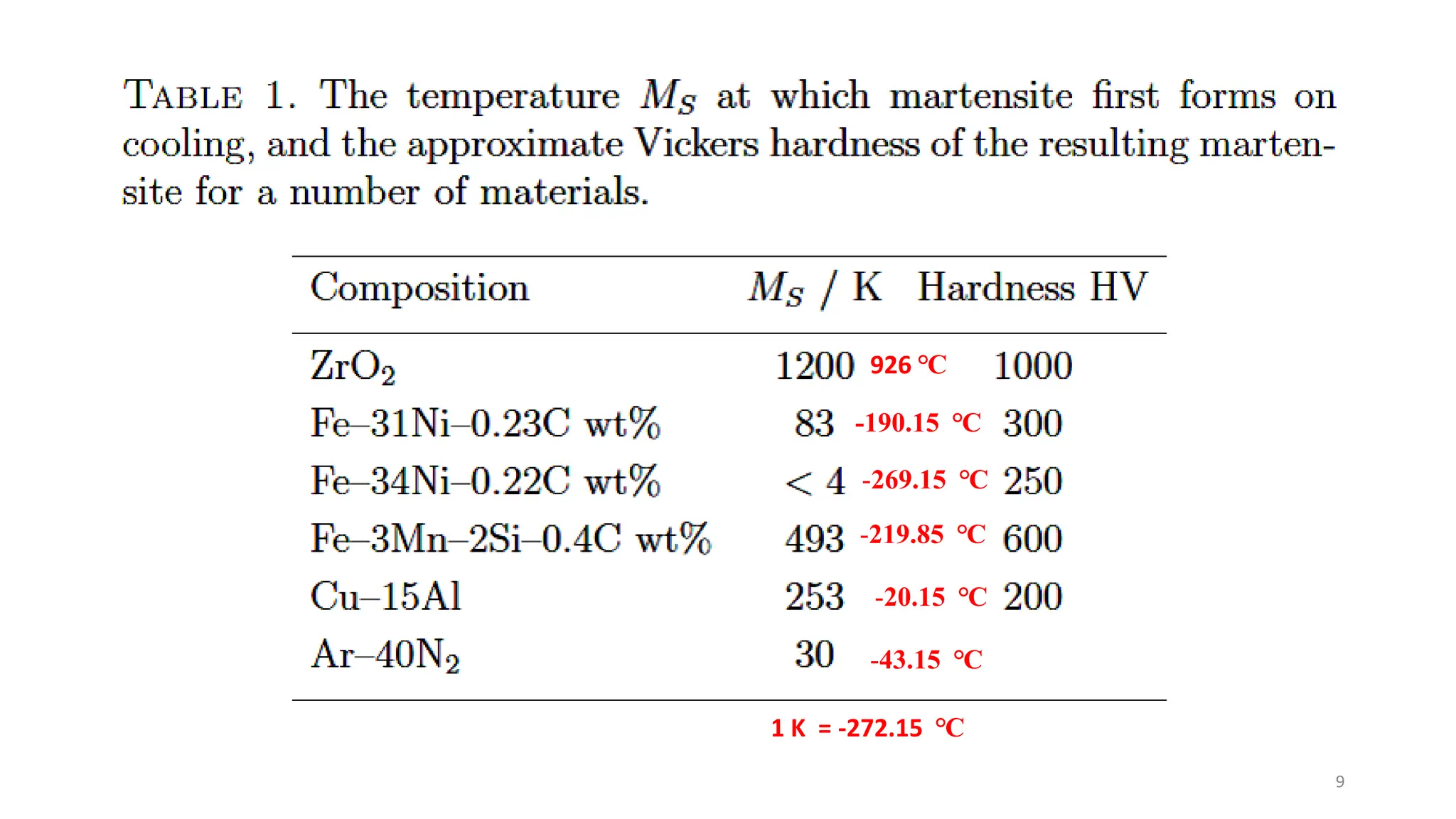
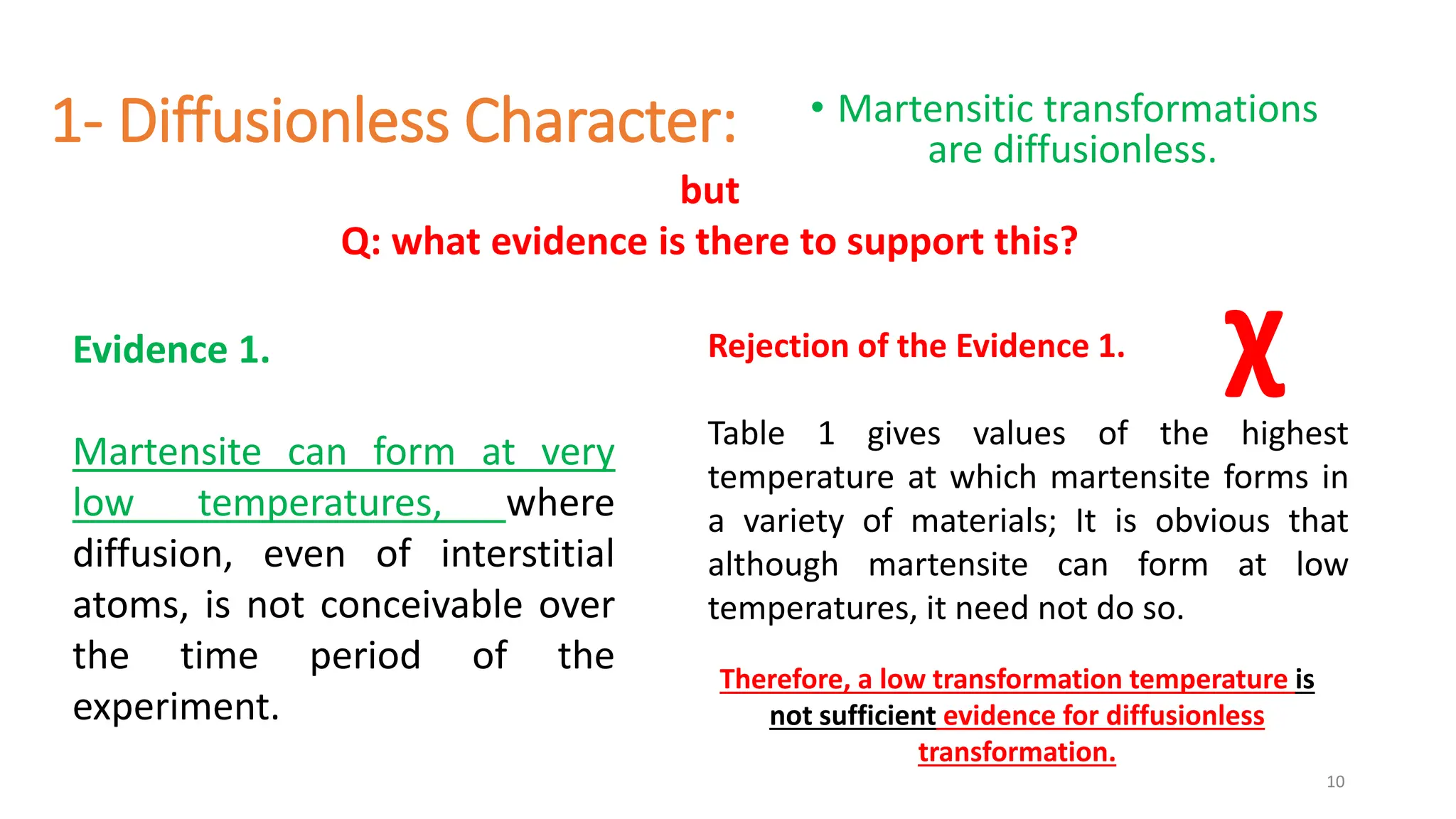
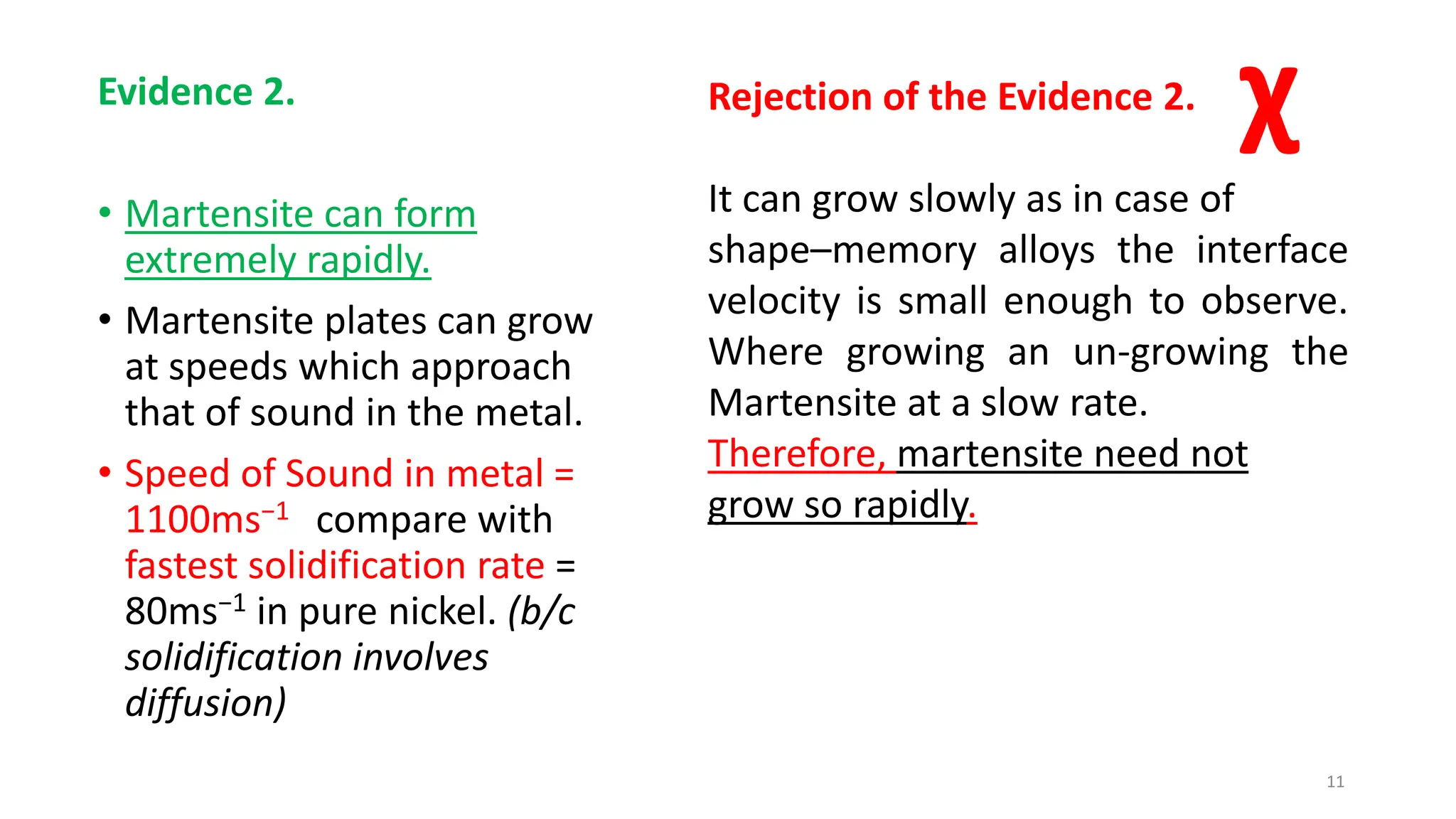
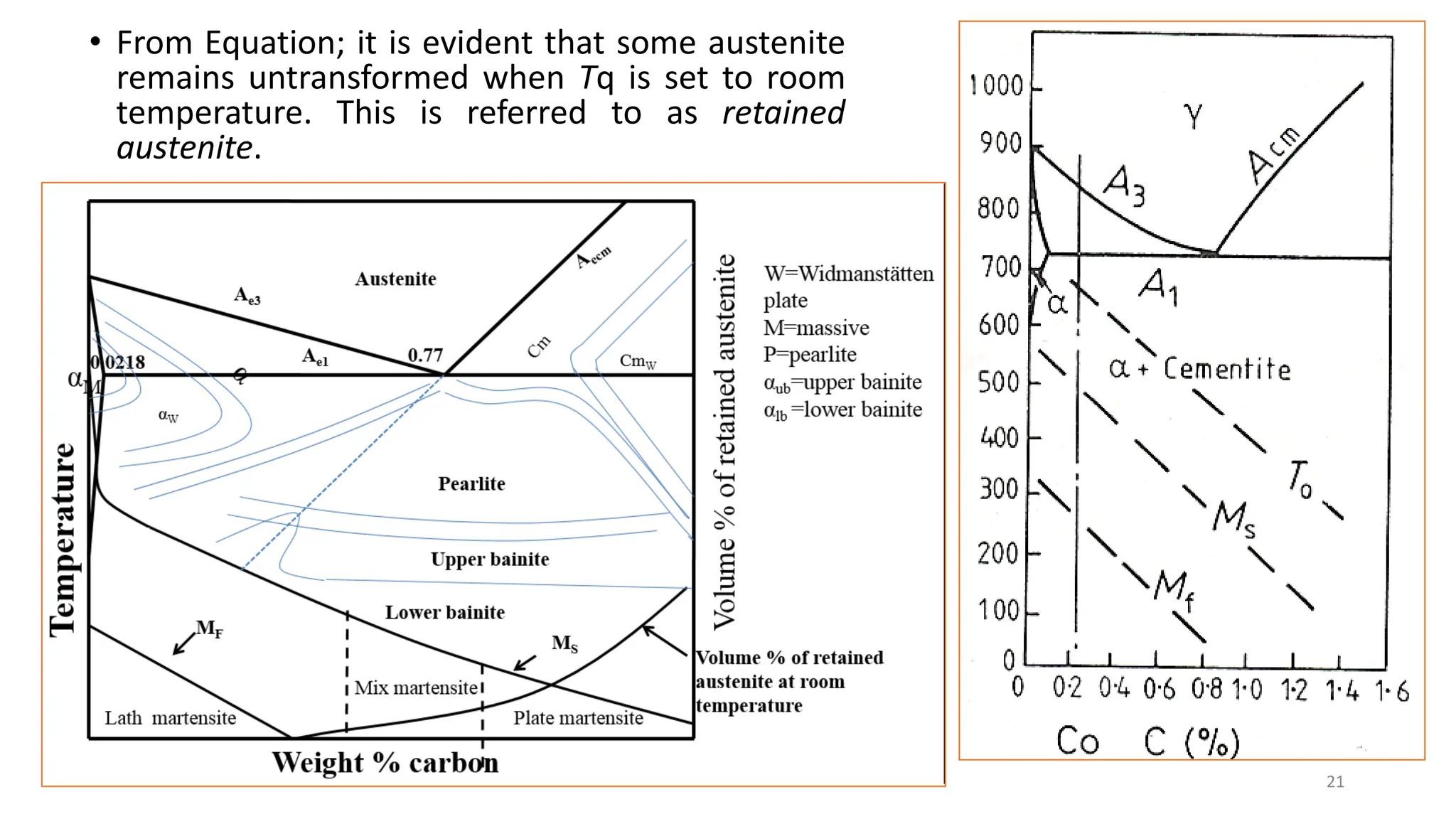
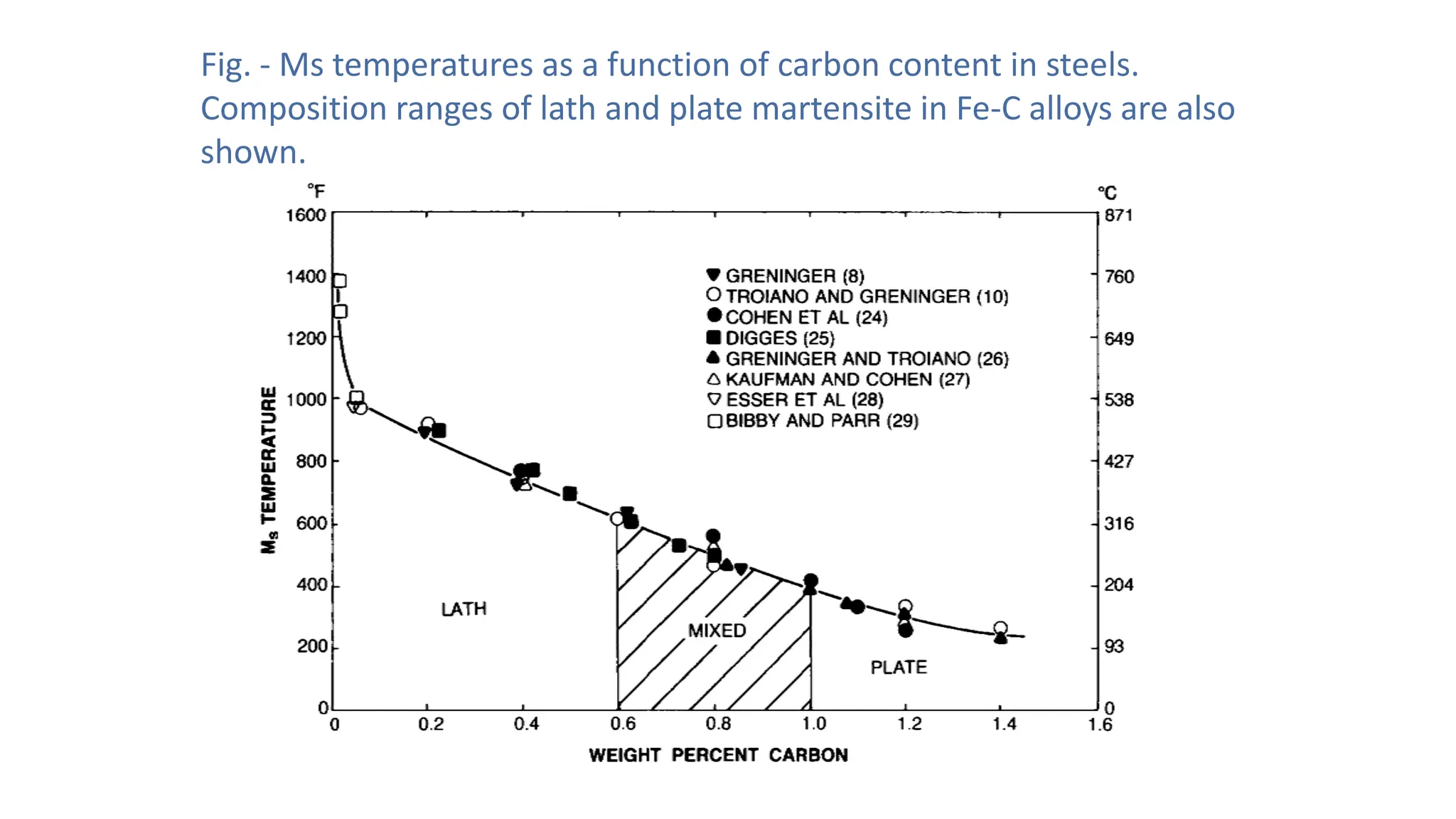
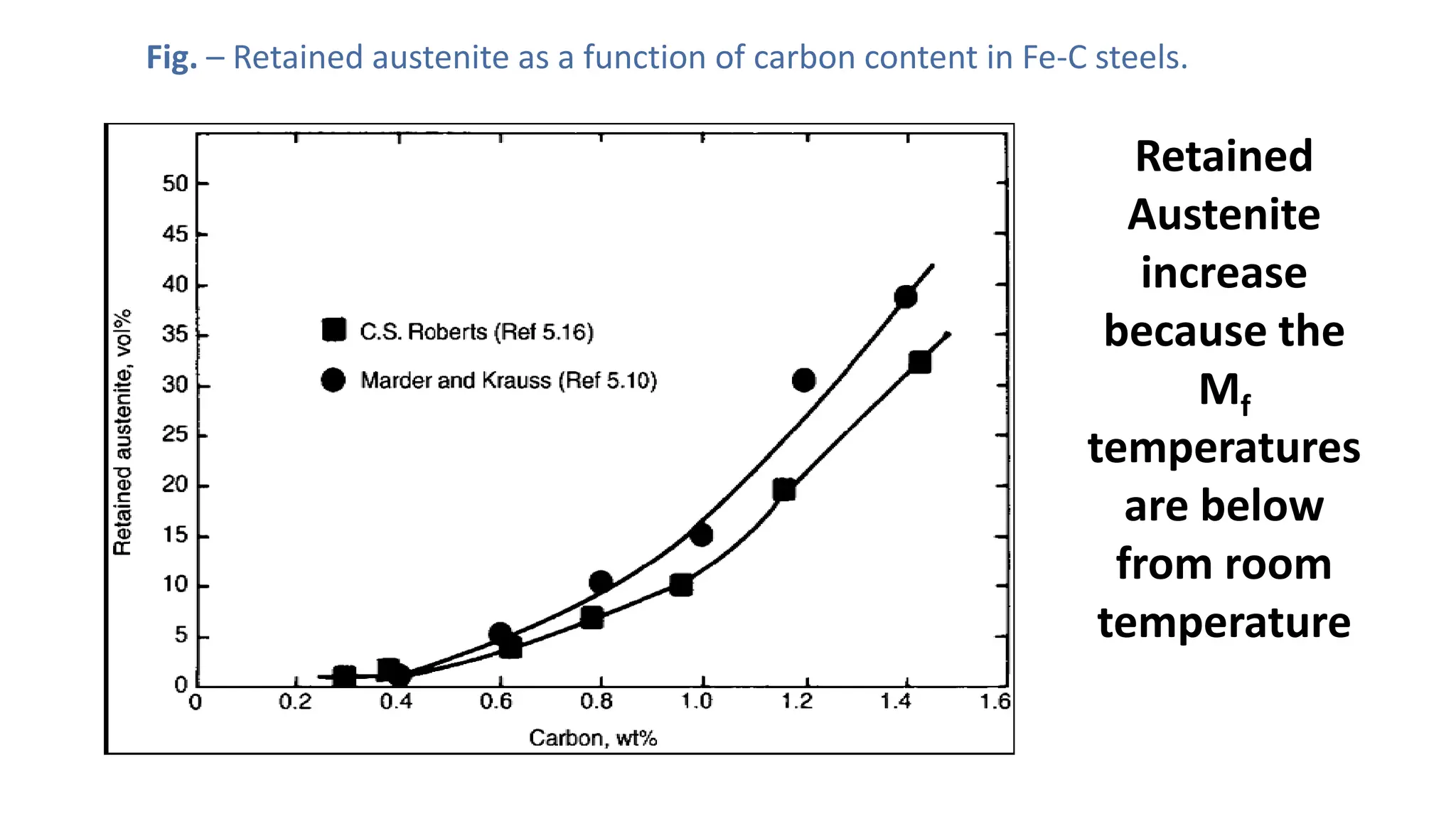
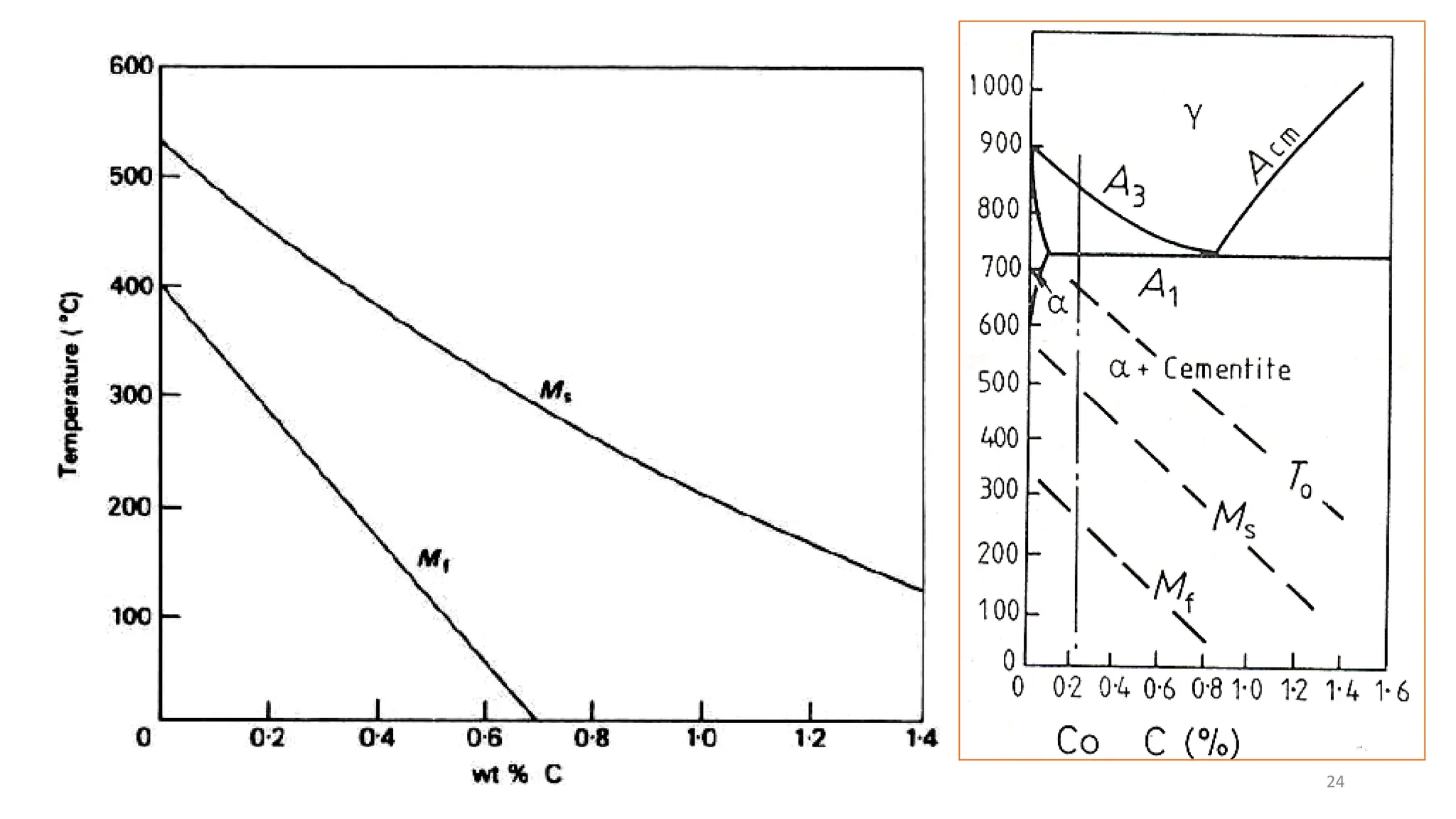
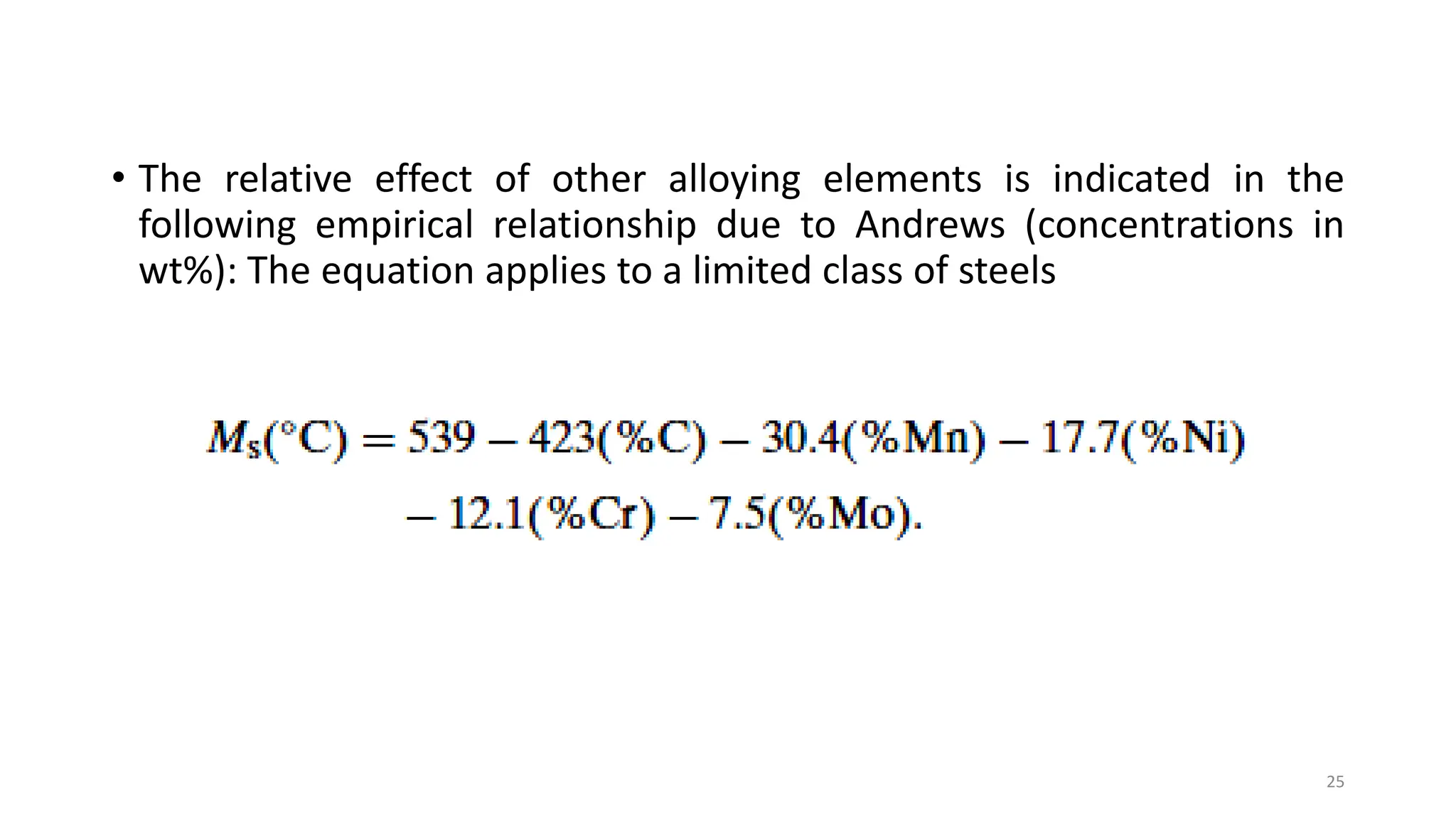
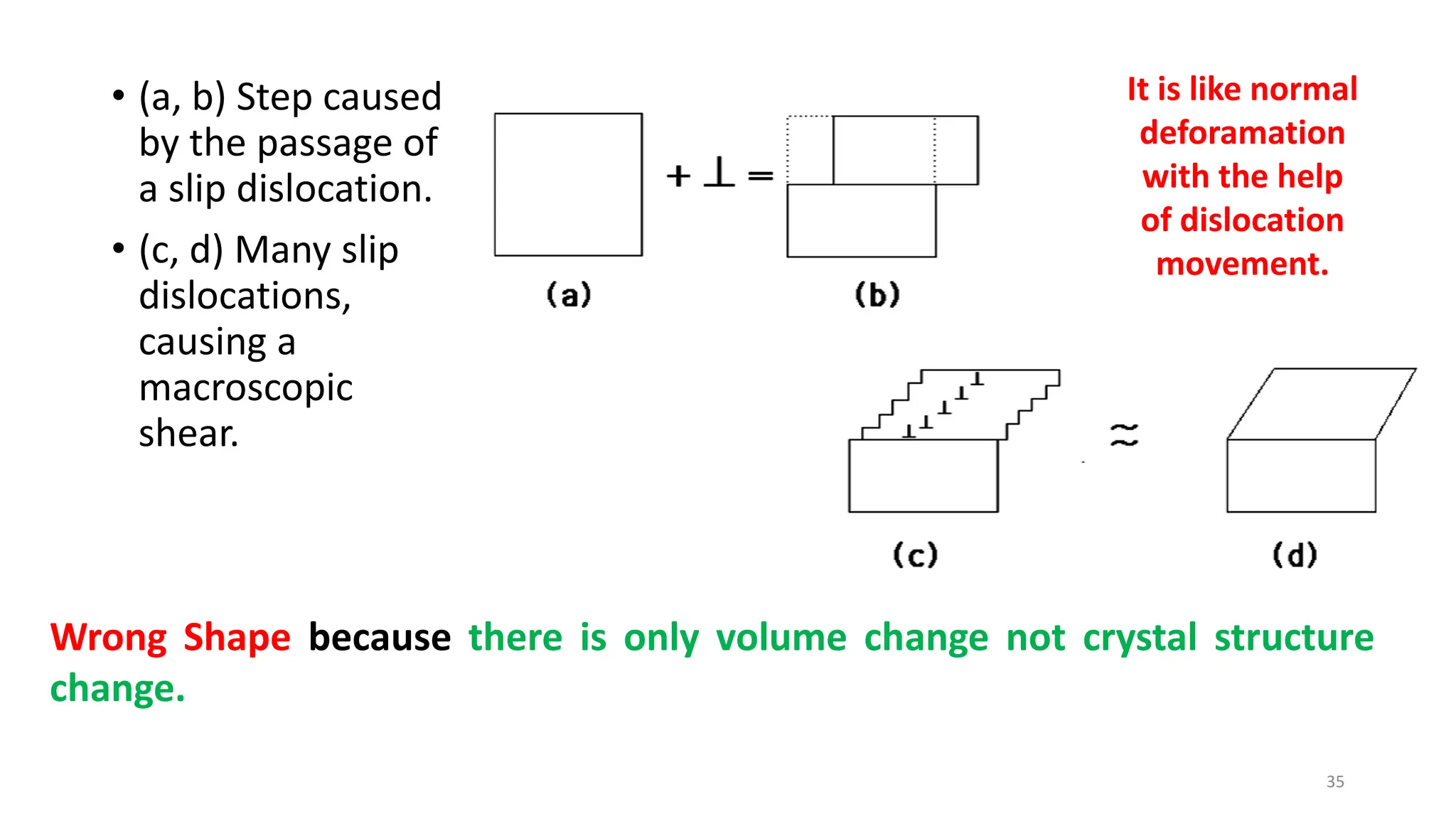
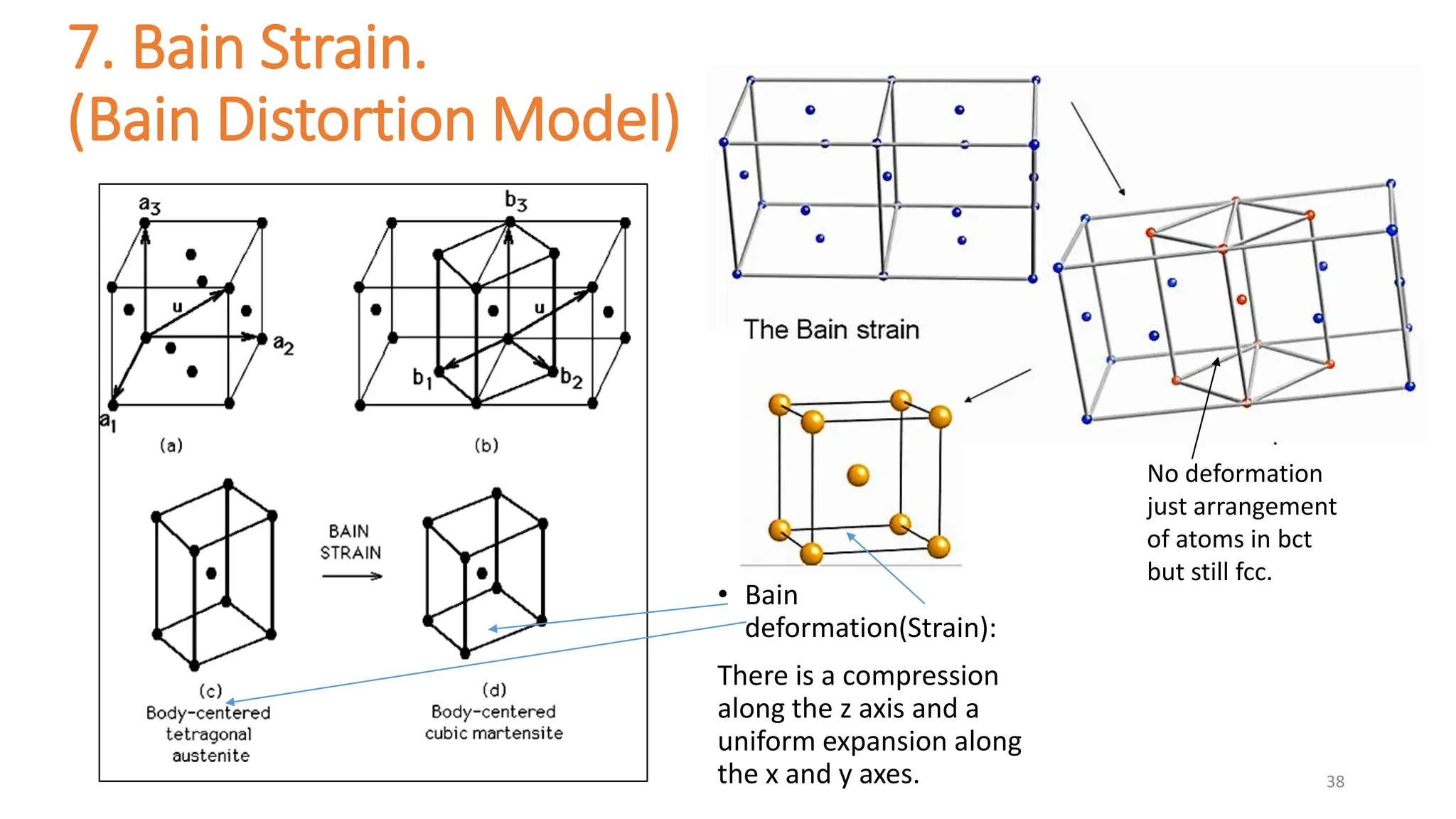
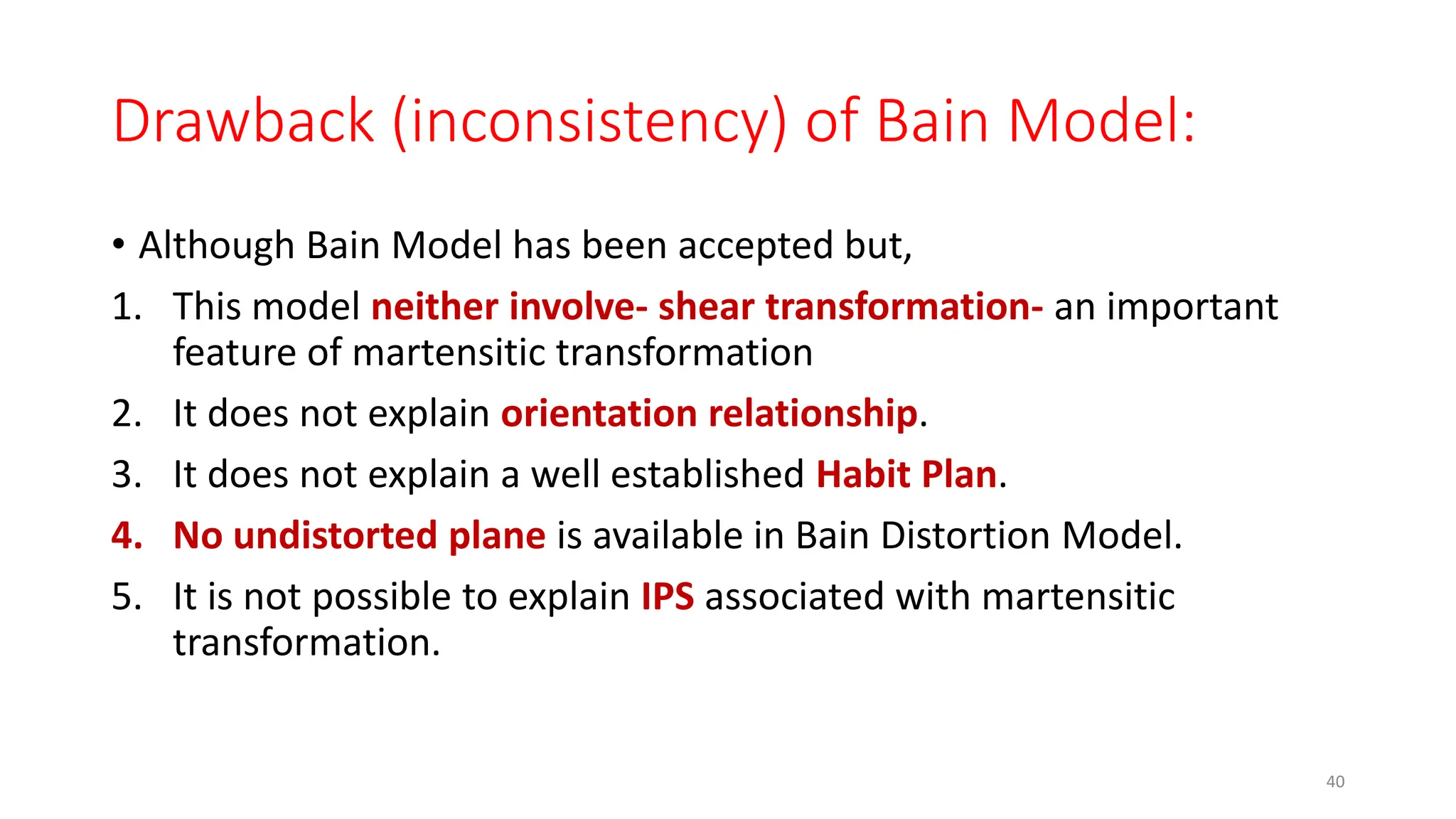
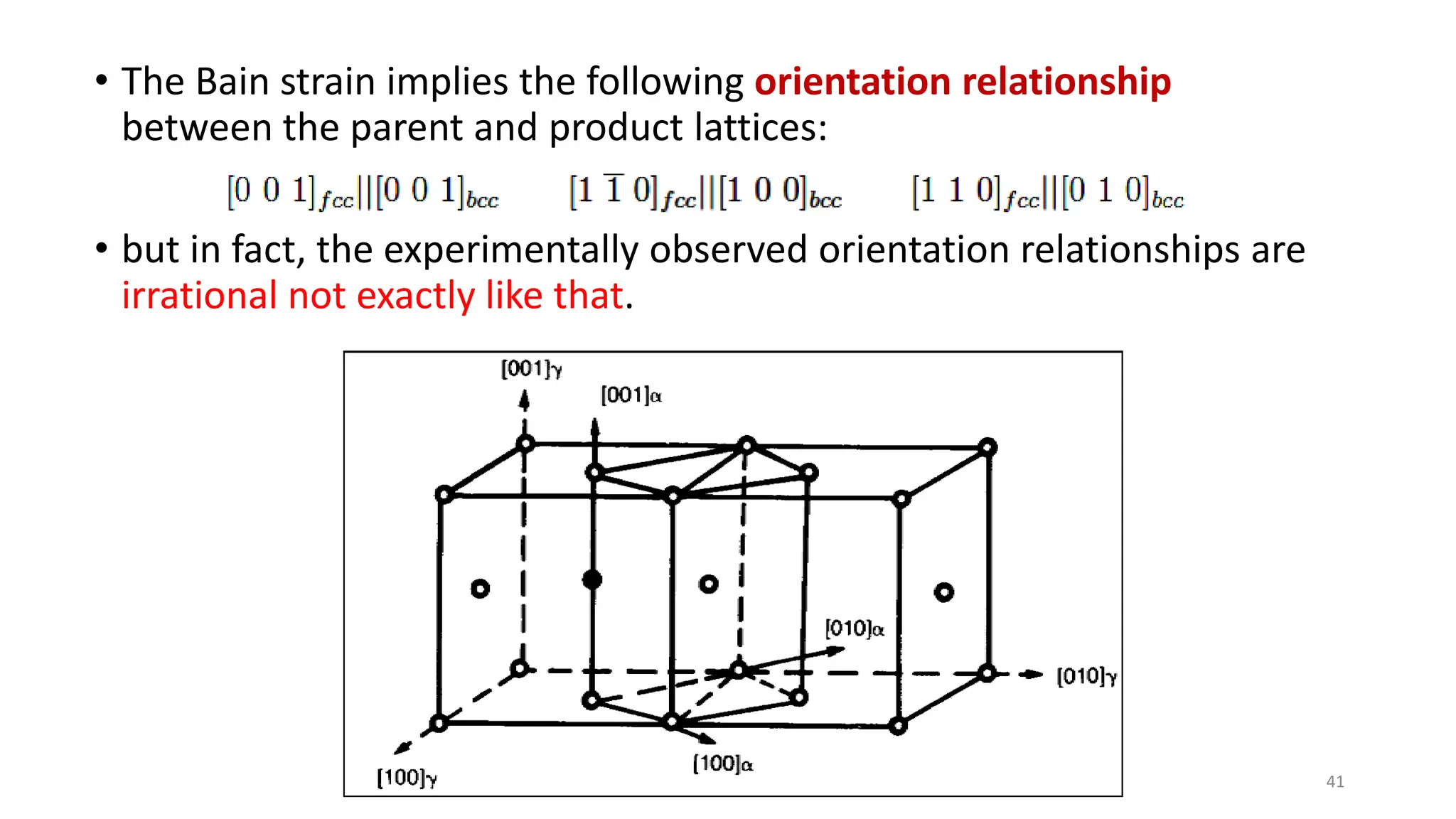
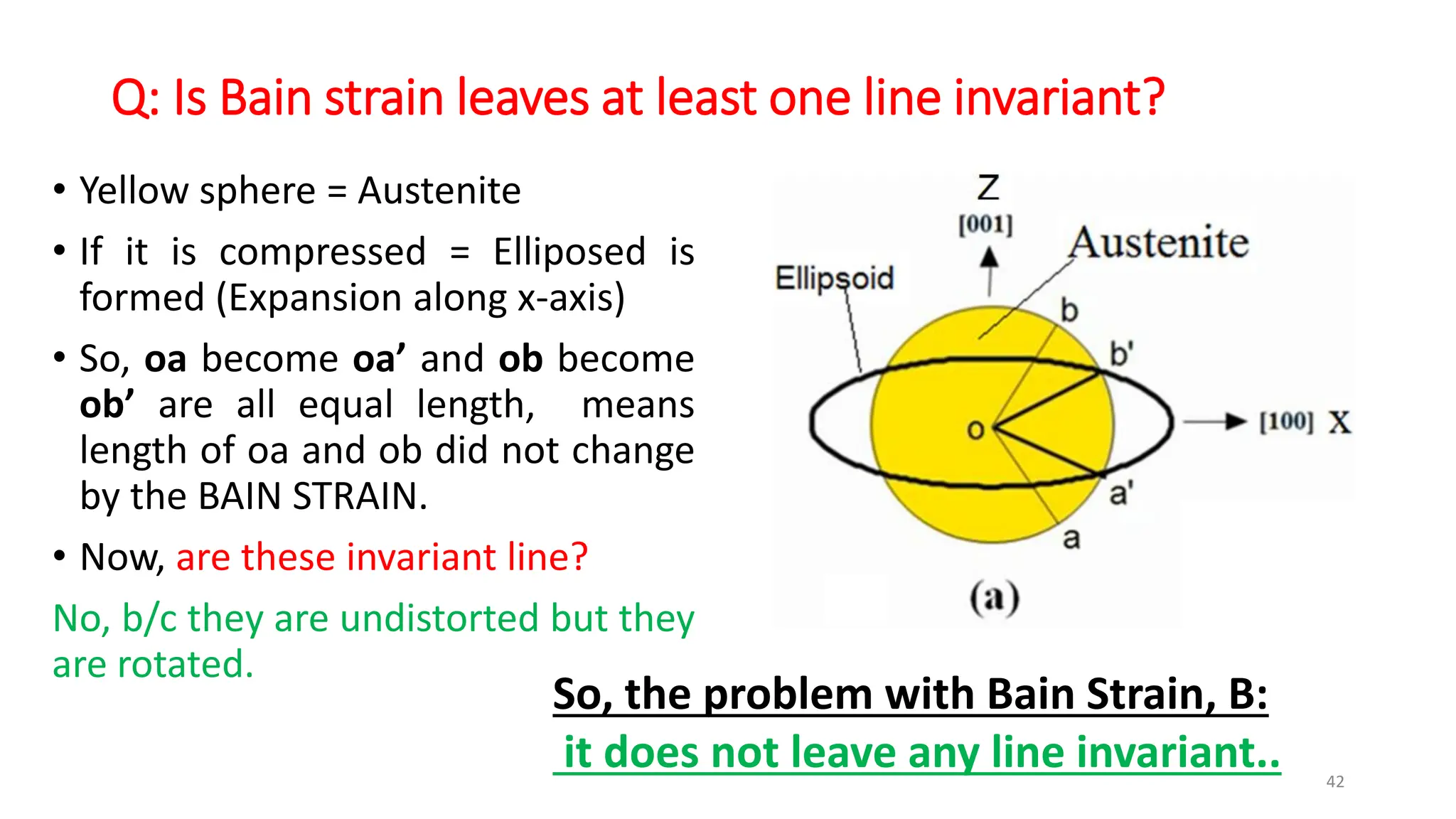
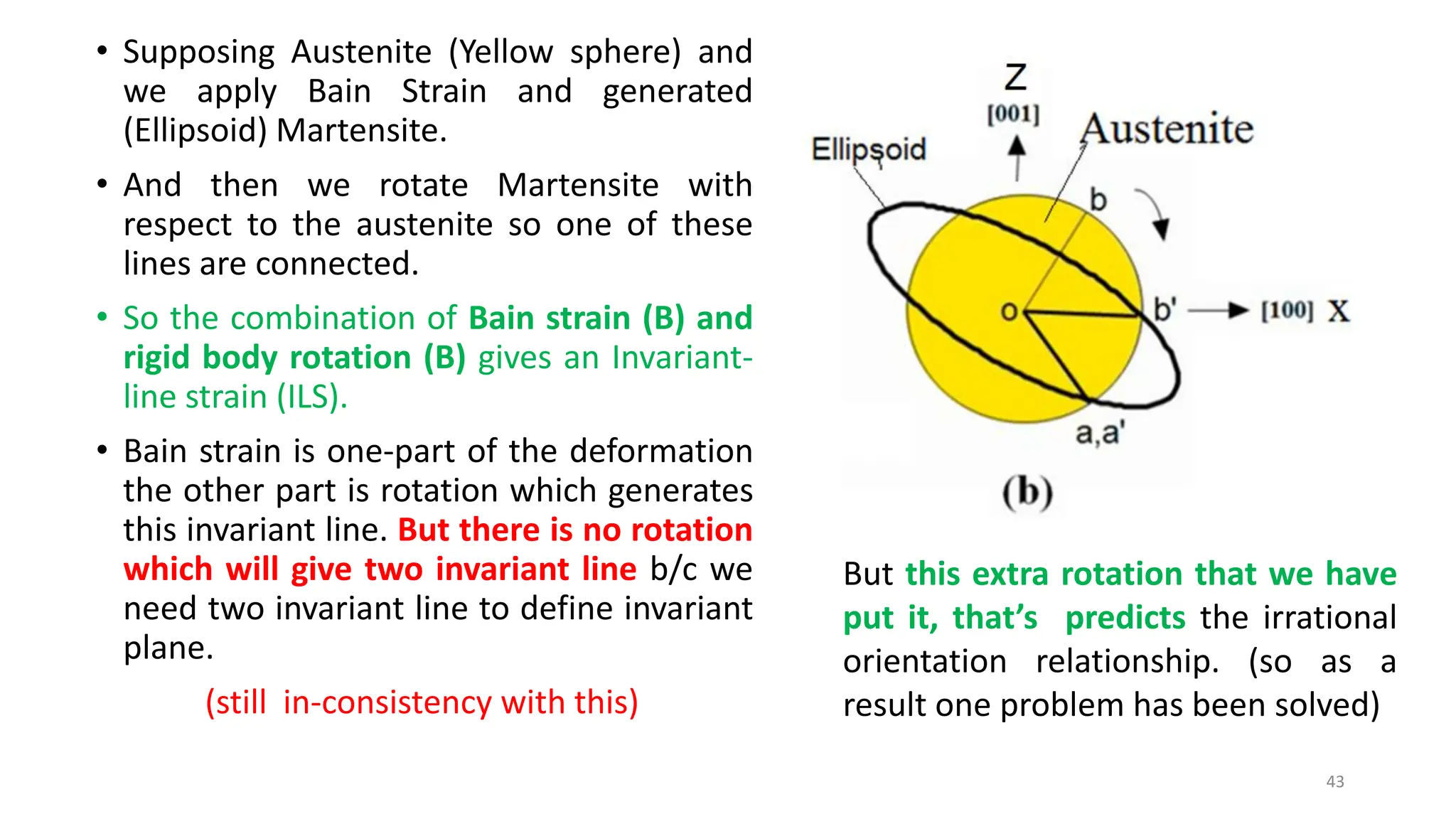
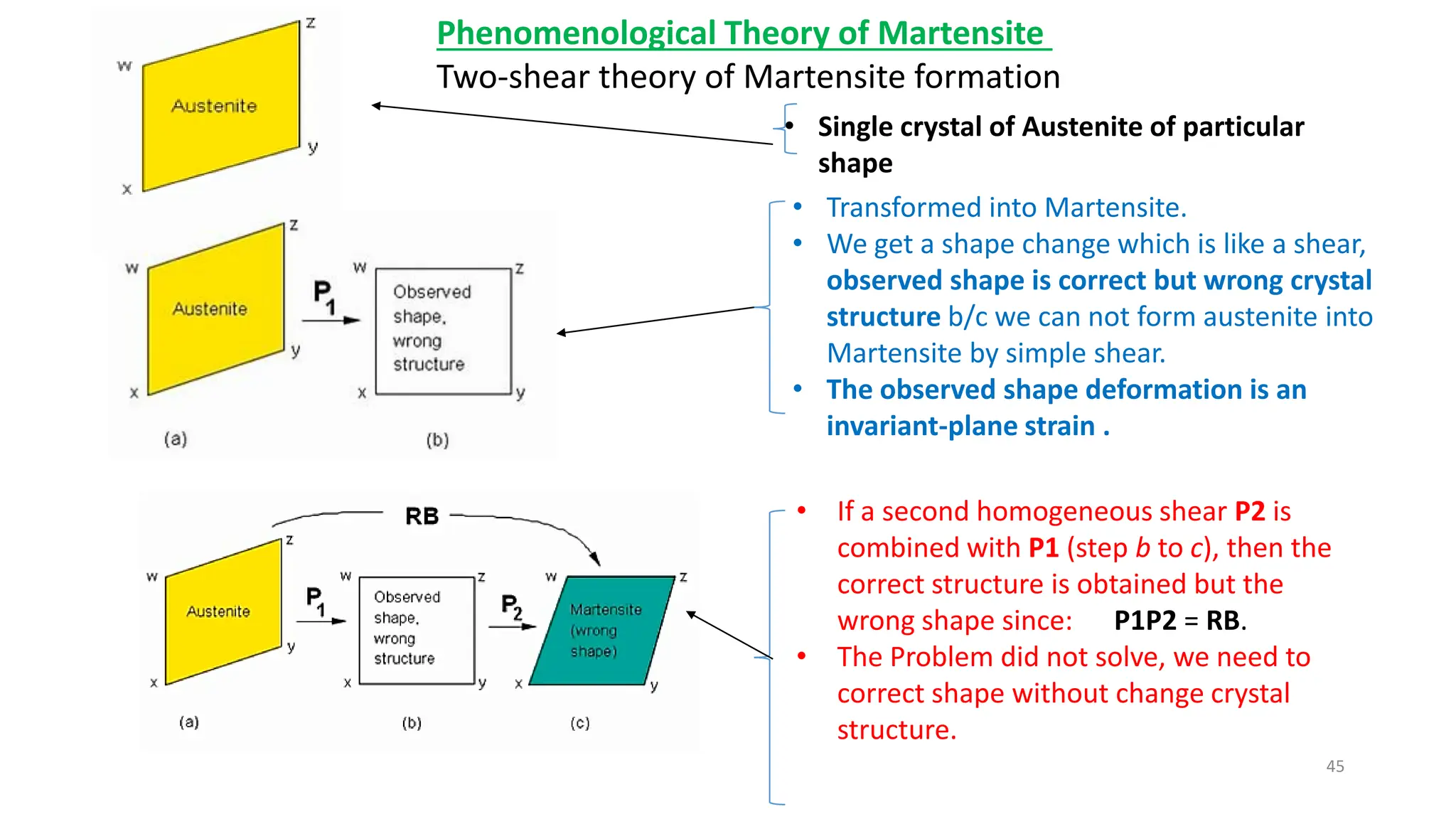
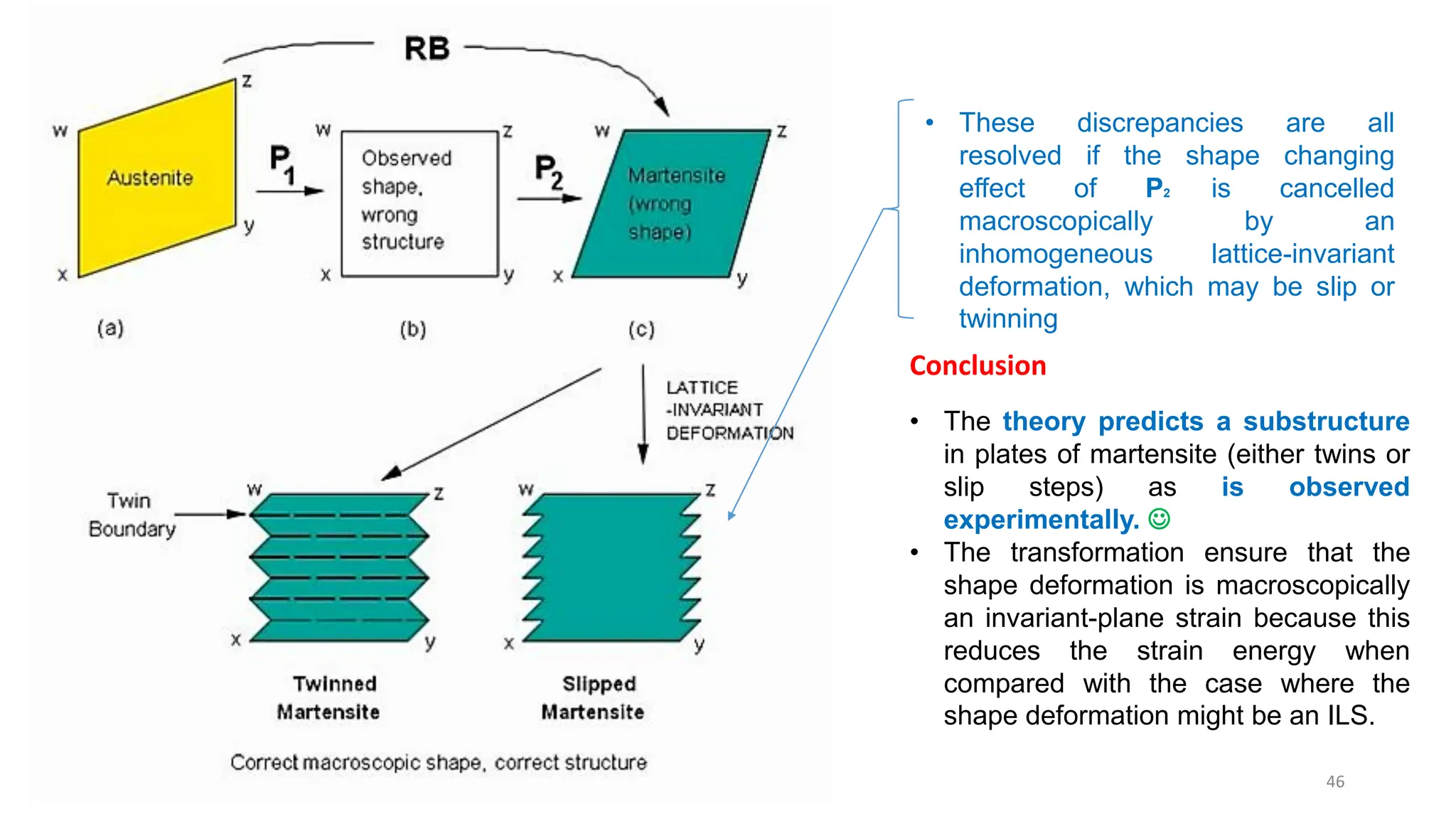
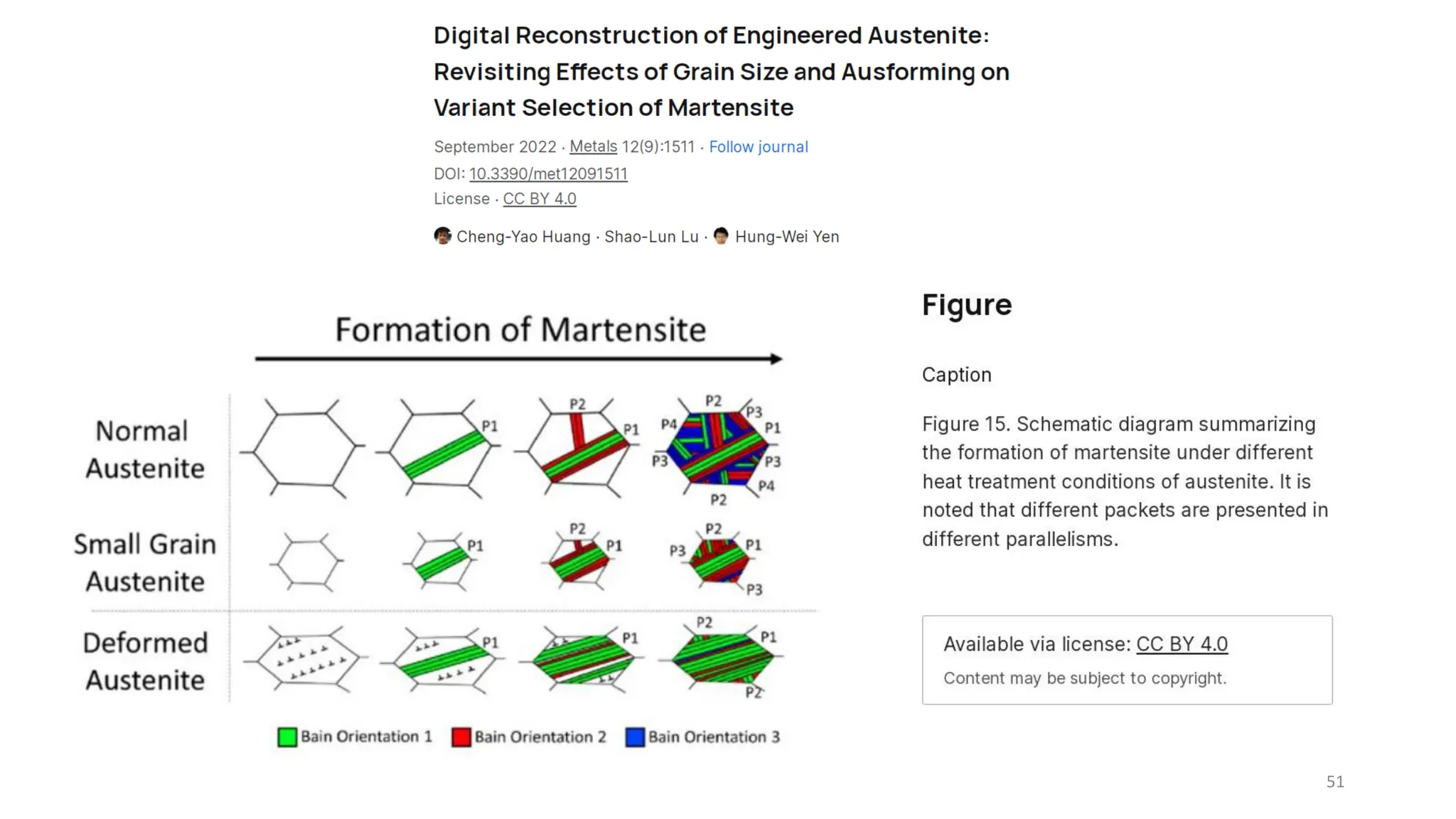
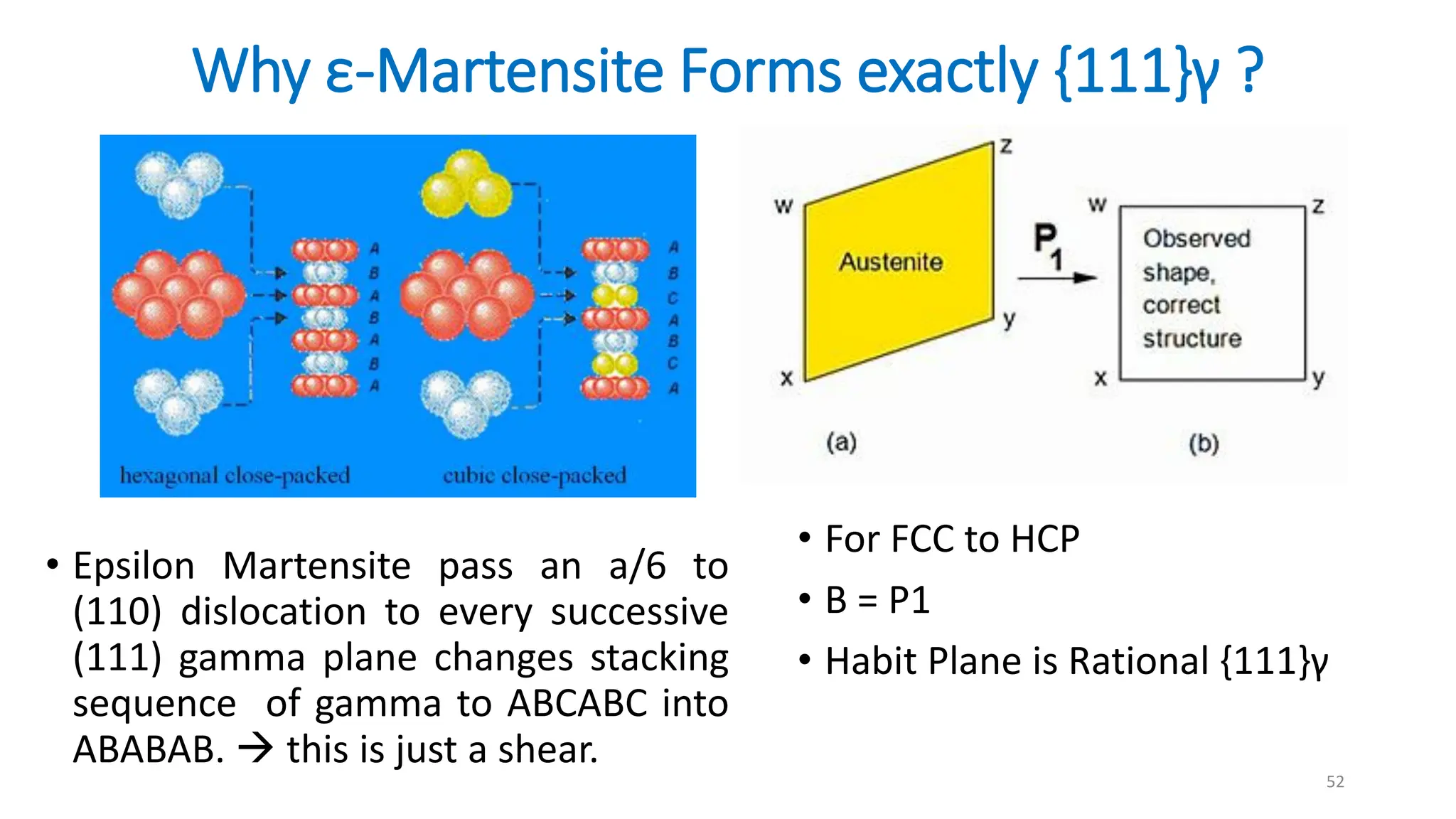
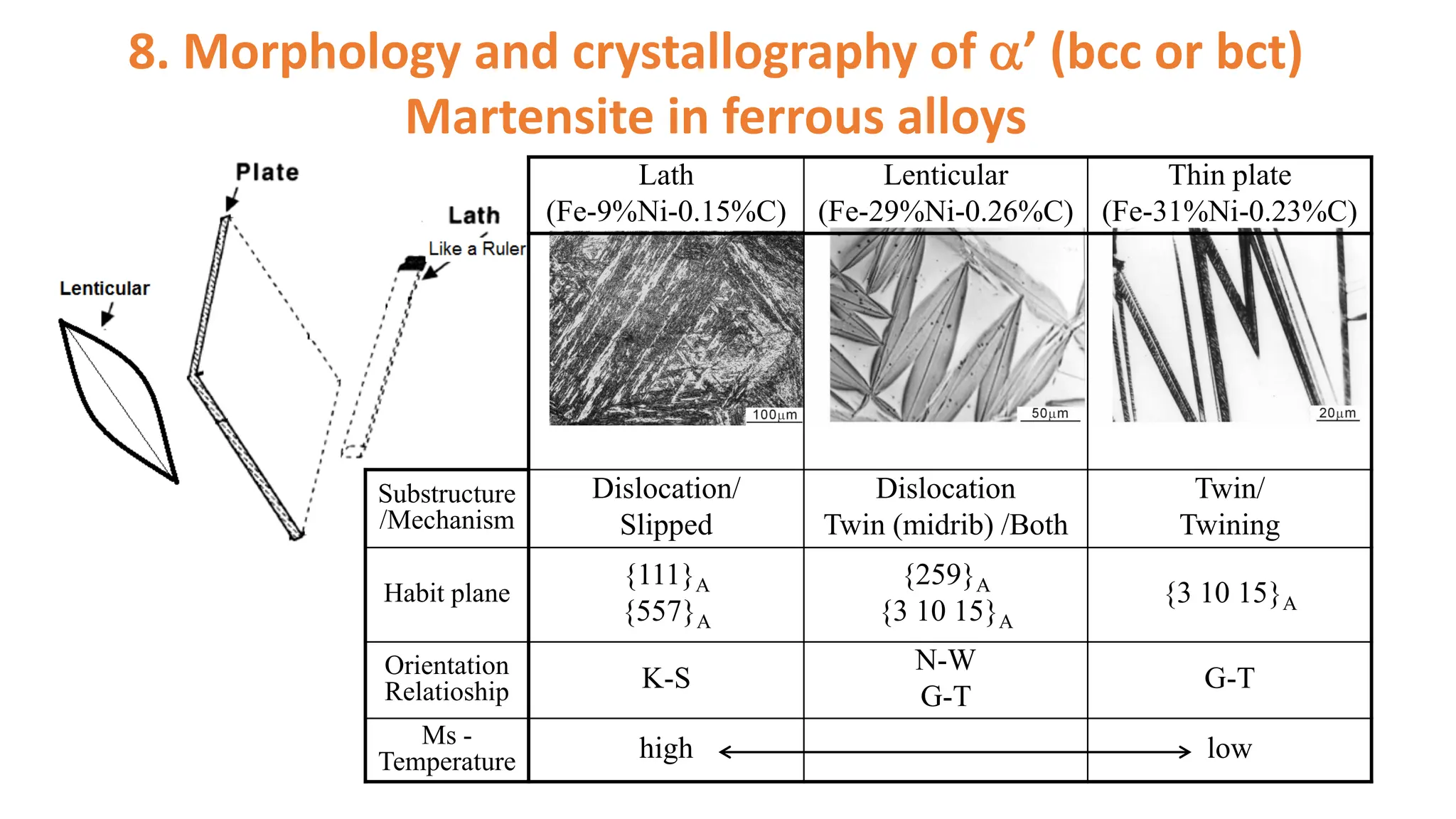
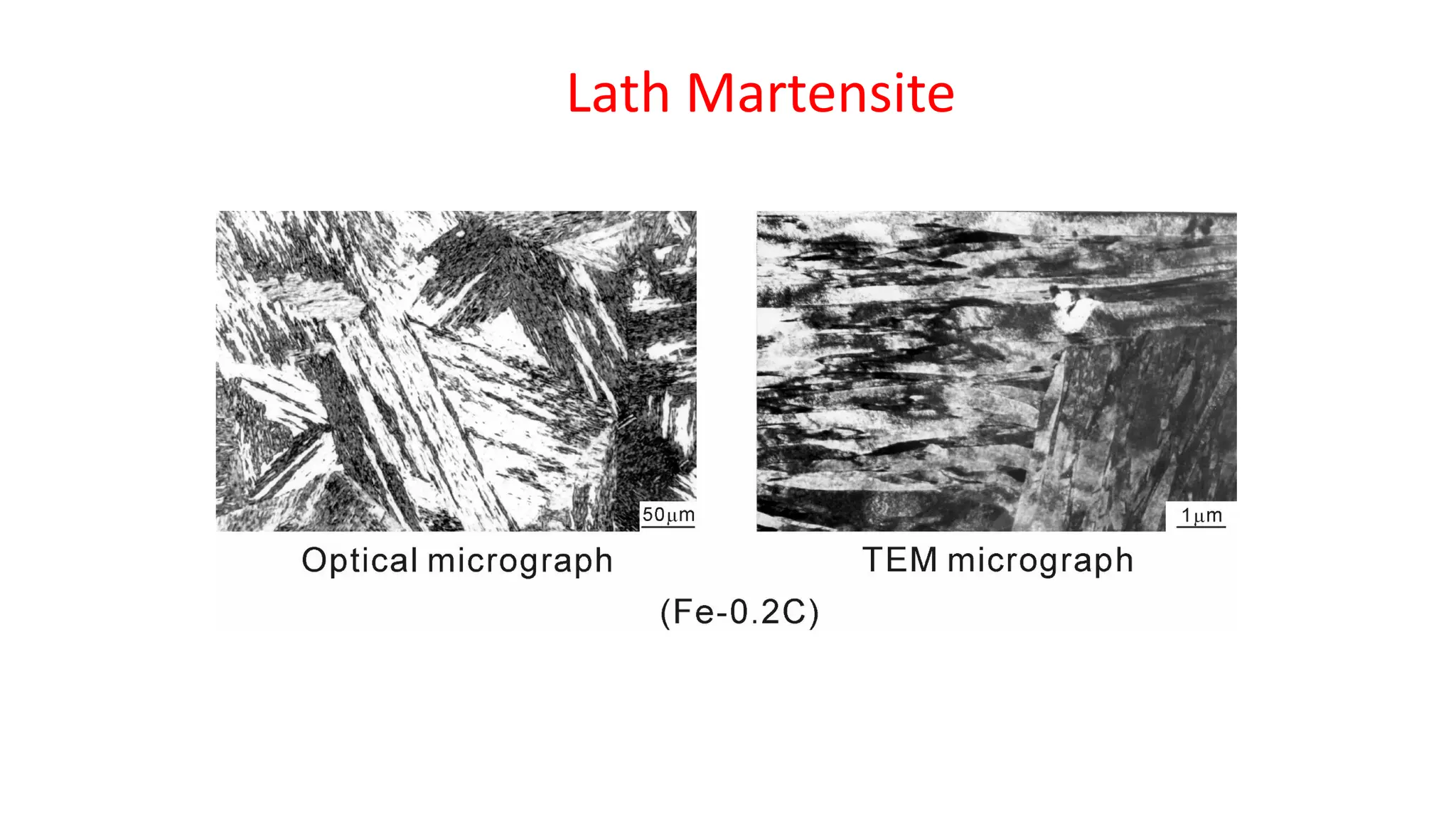
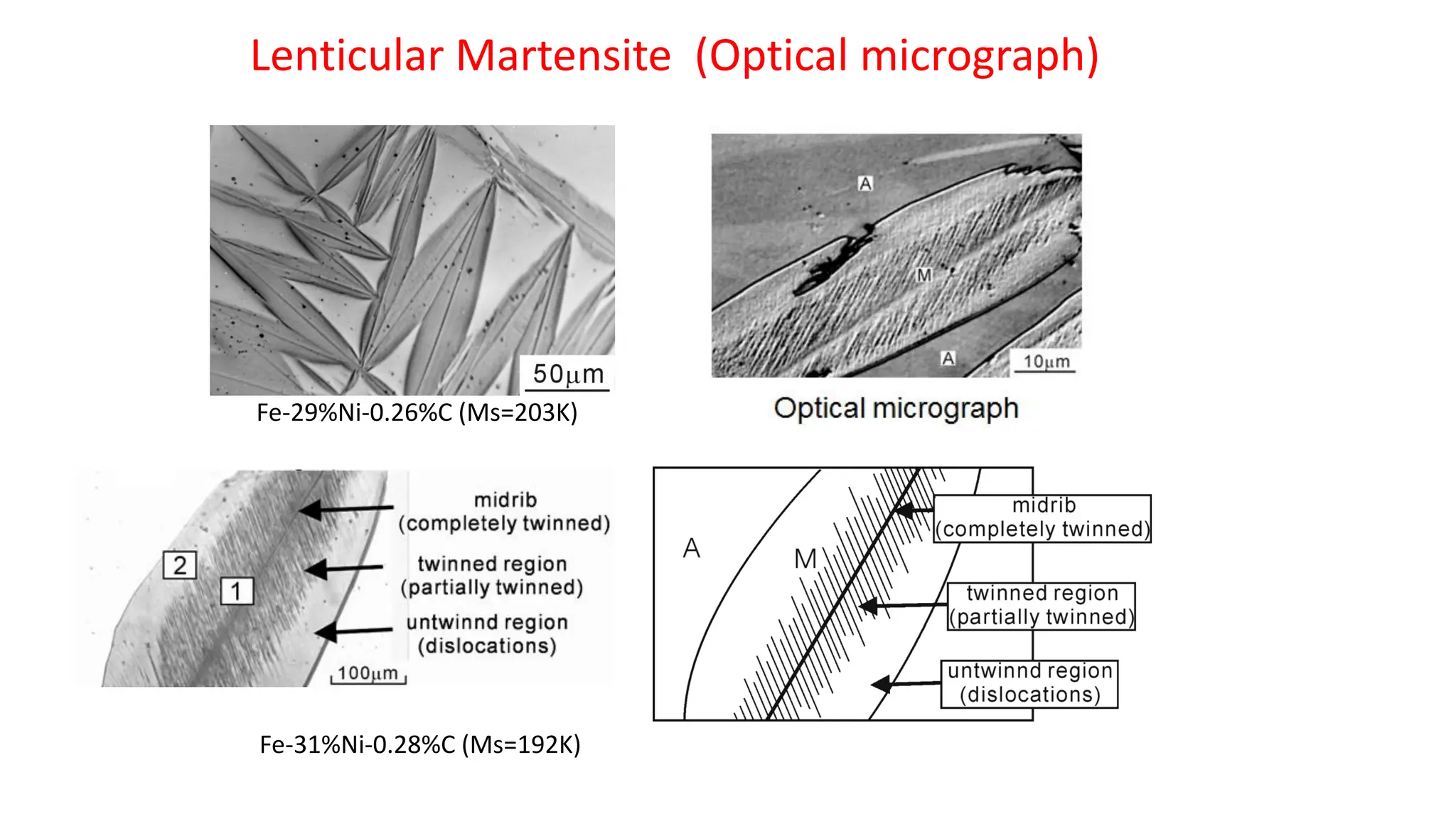
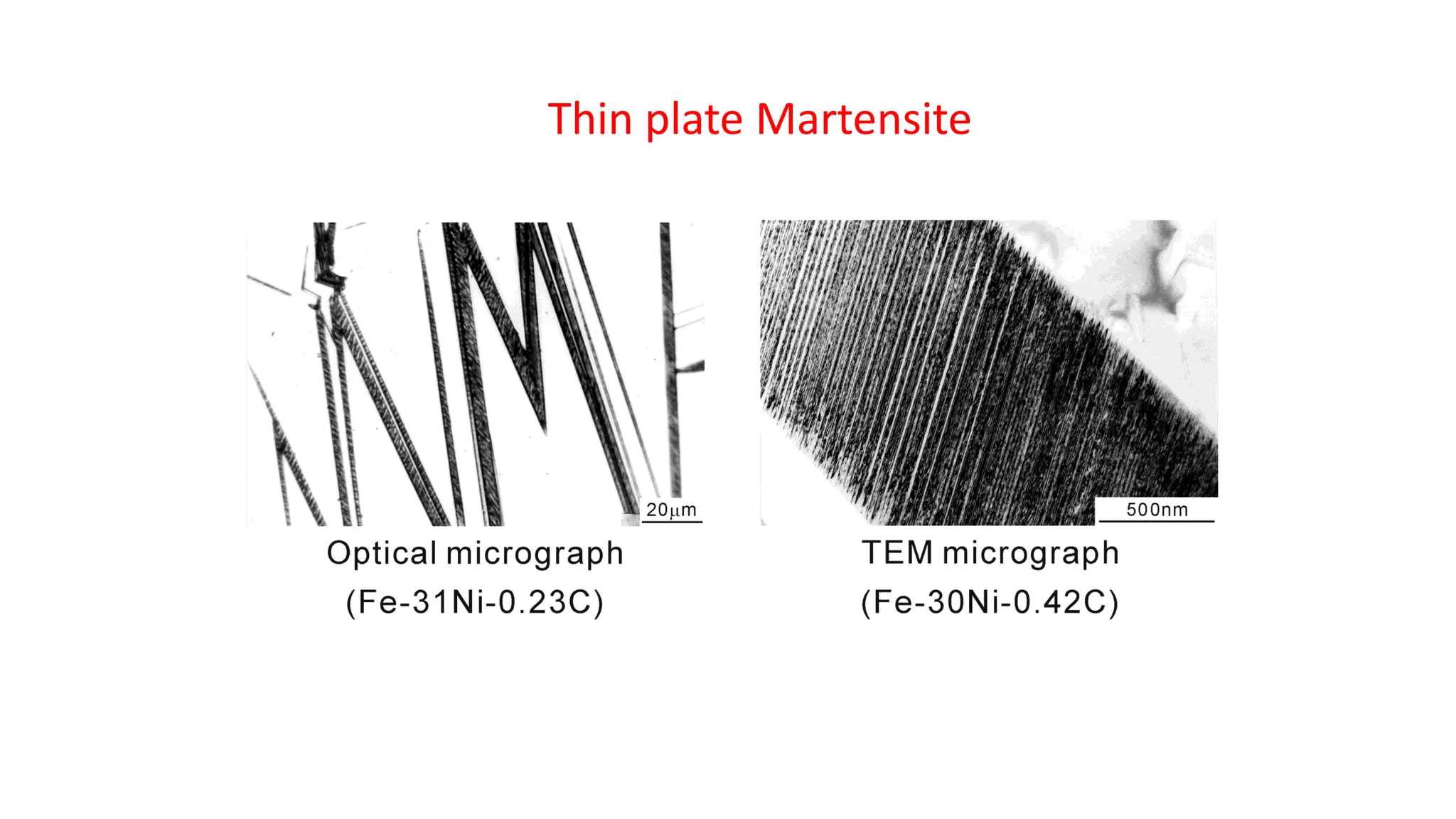
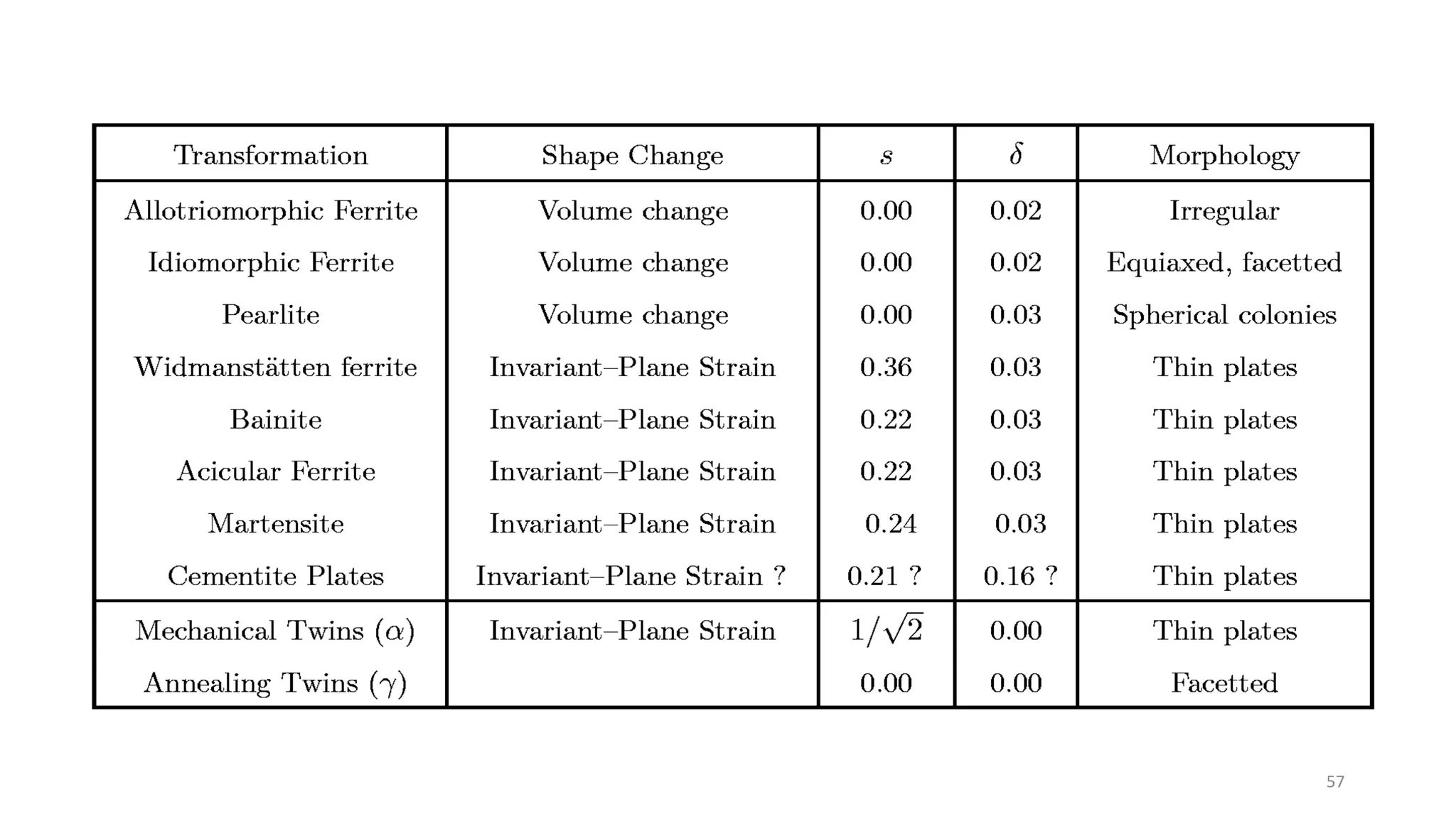
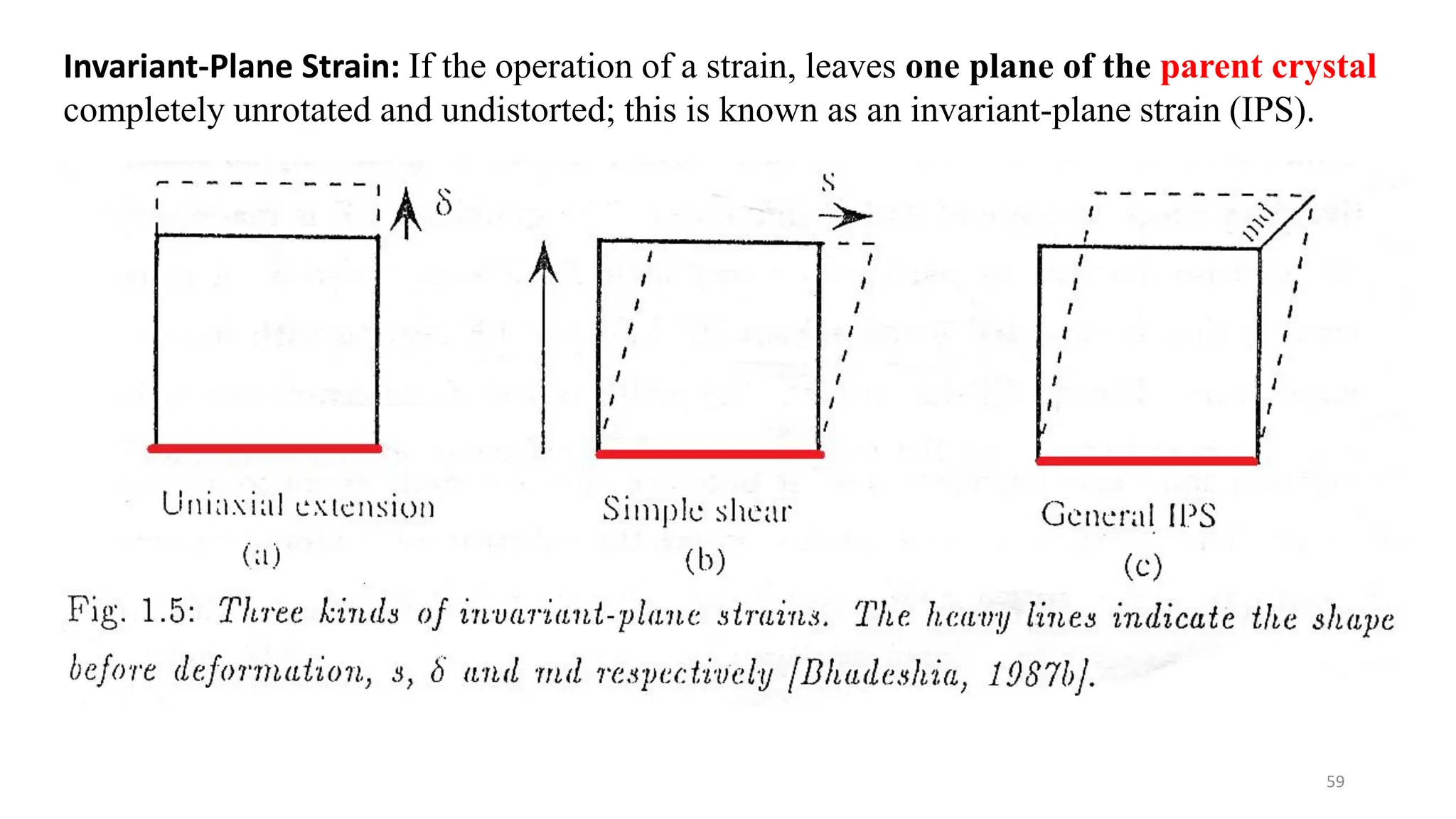
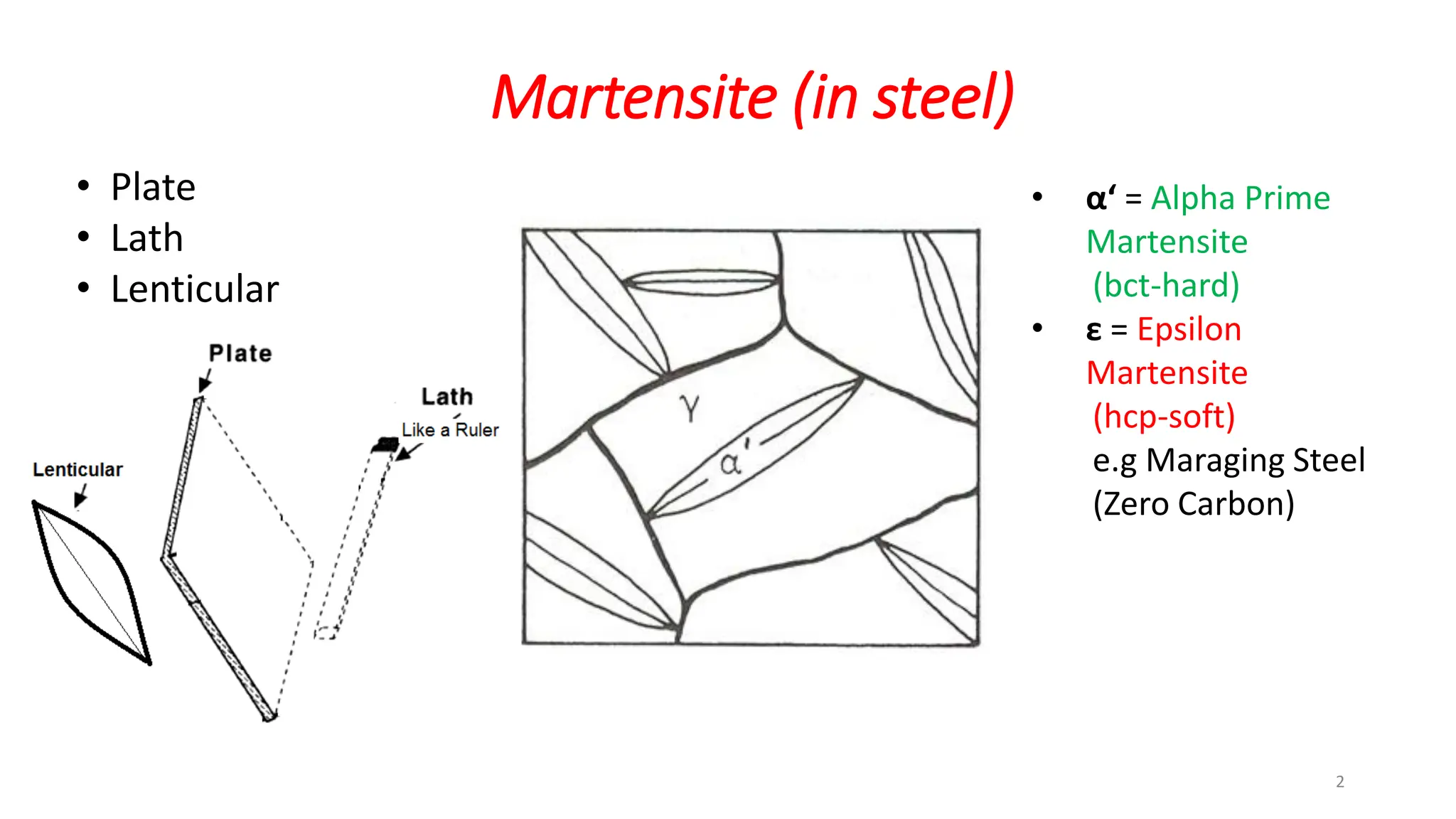
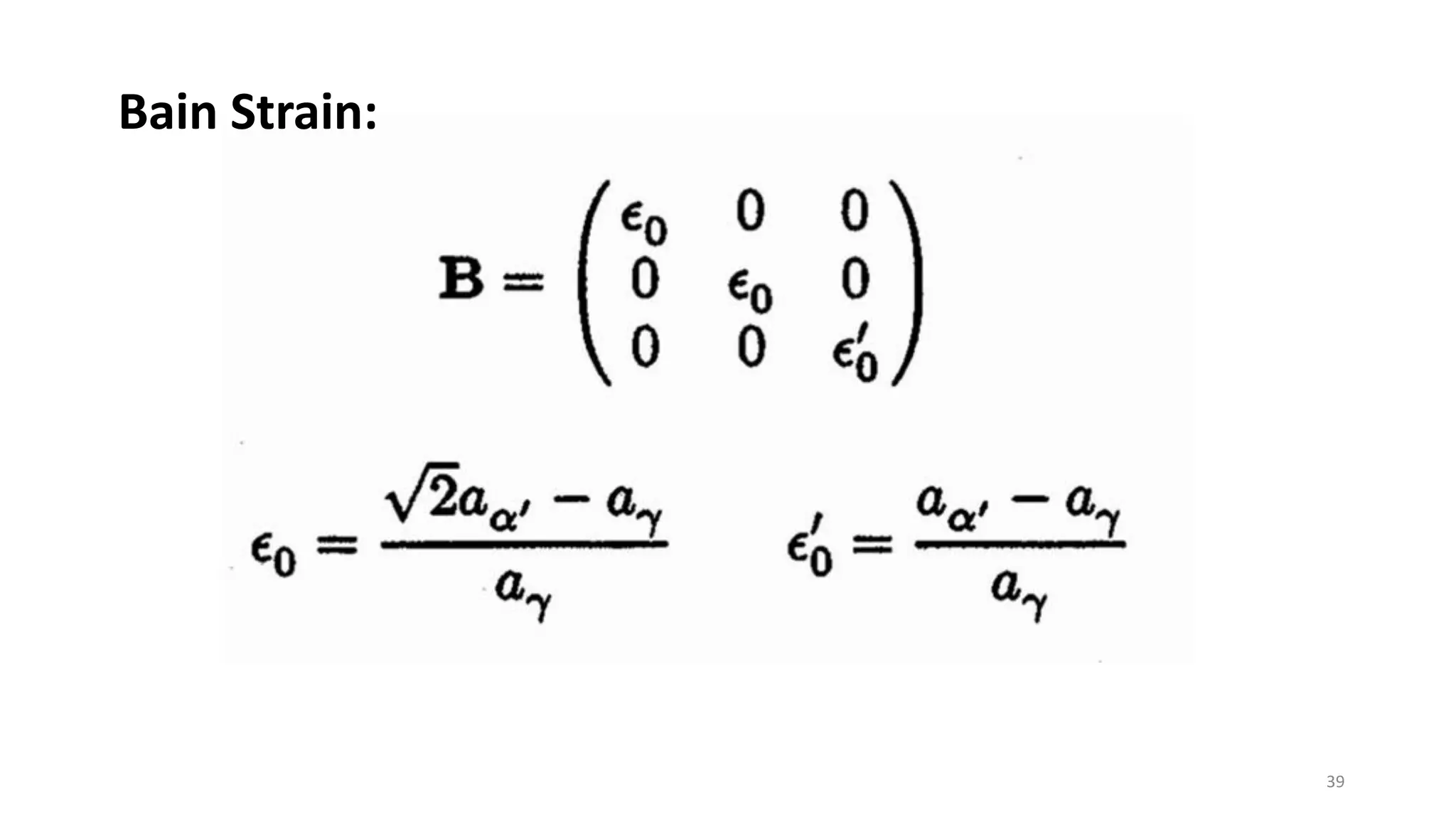1. Martensite forms via a diffusionless, displacive transformation in which the crystal structure changes rapidly without atomic diffusion.
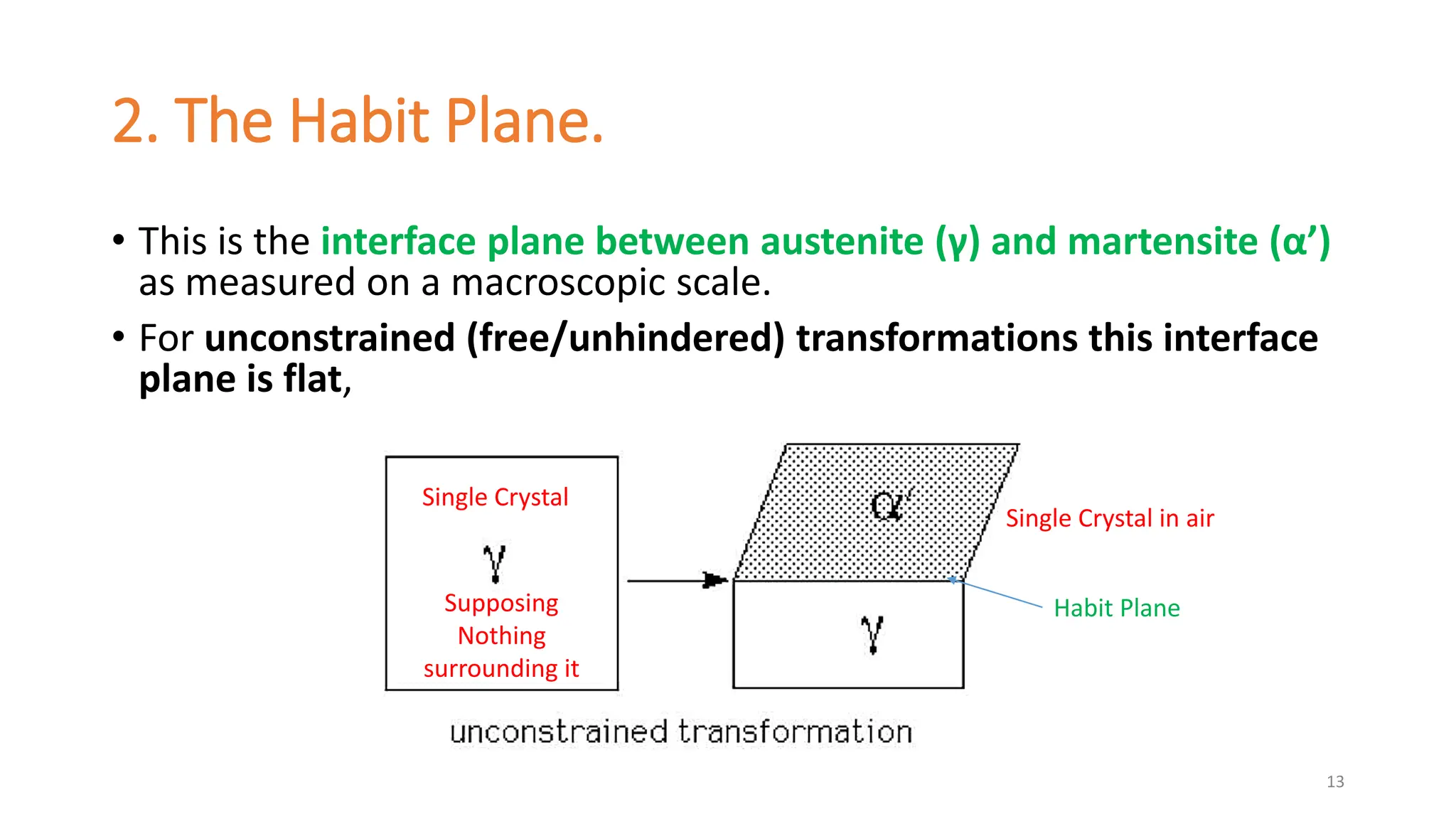
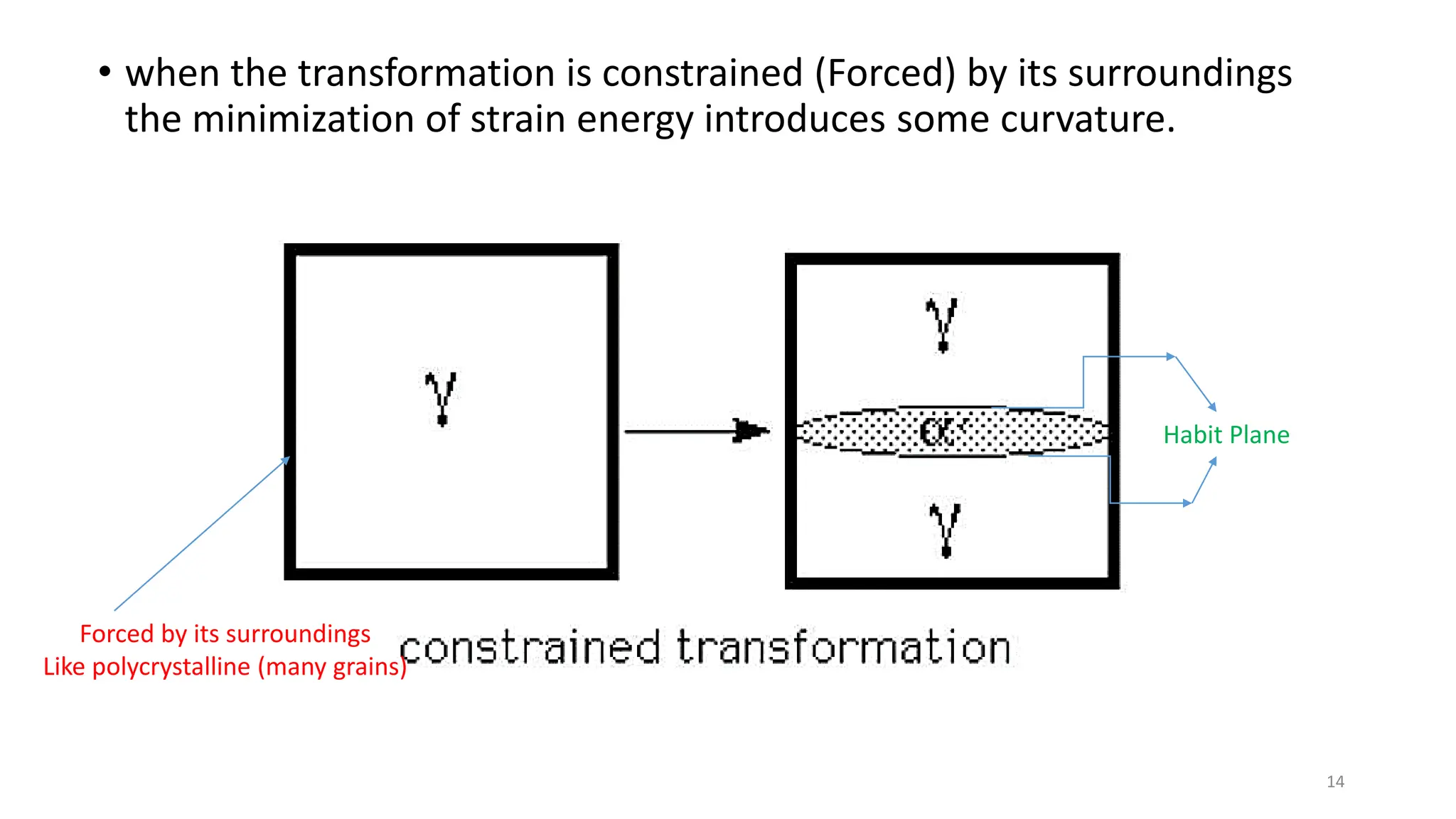
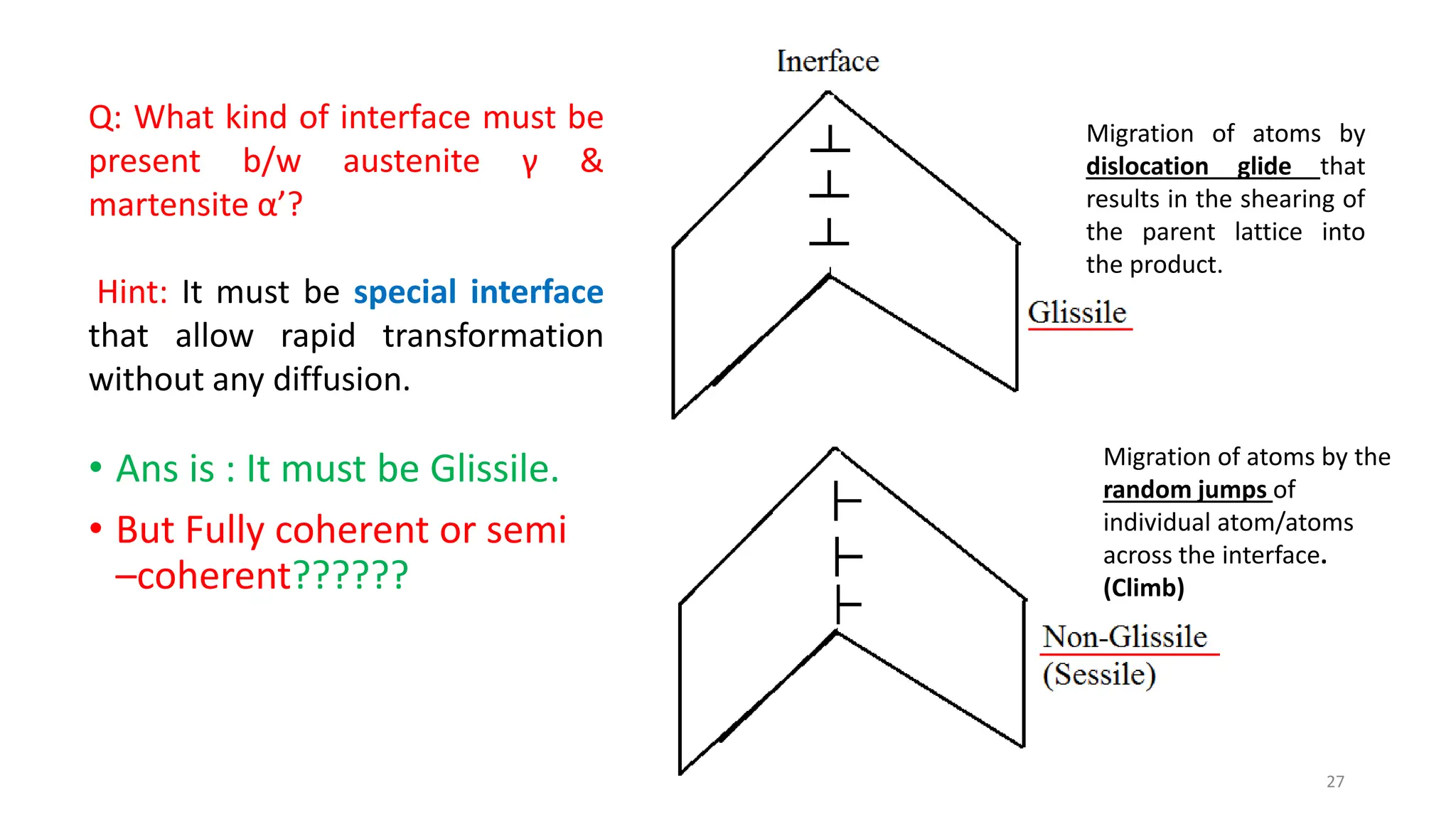
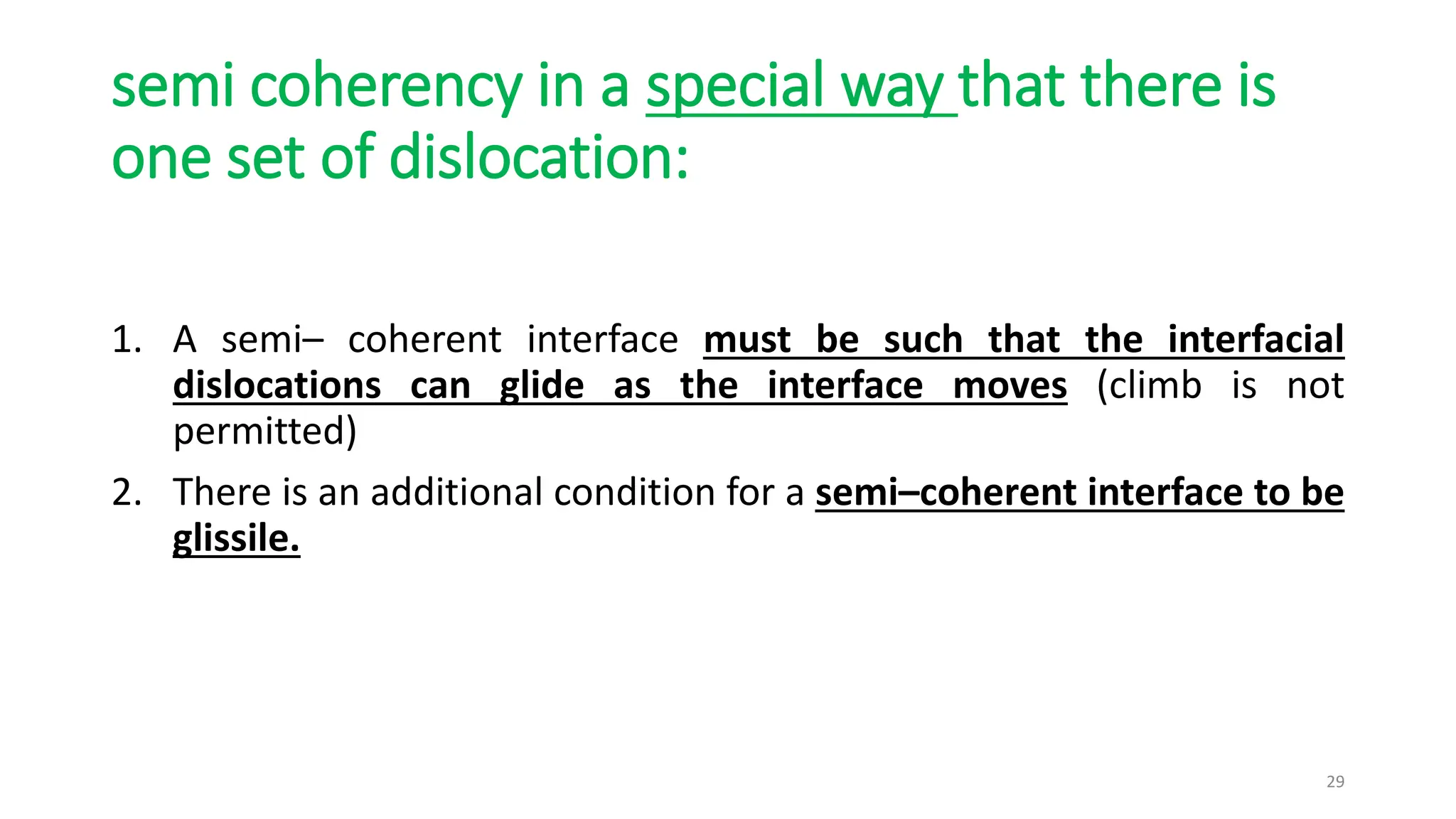
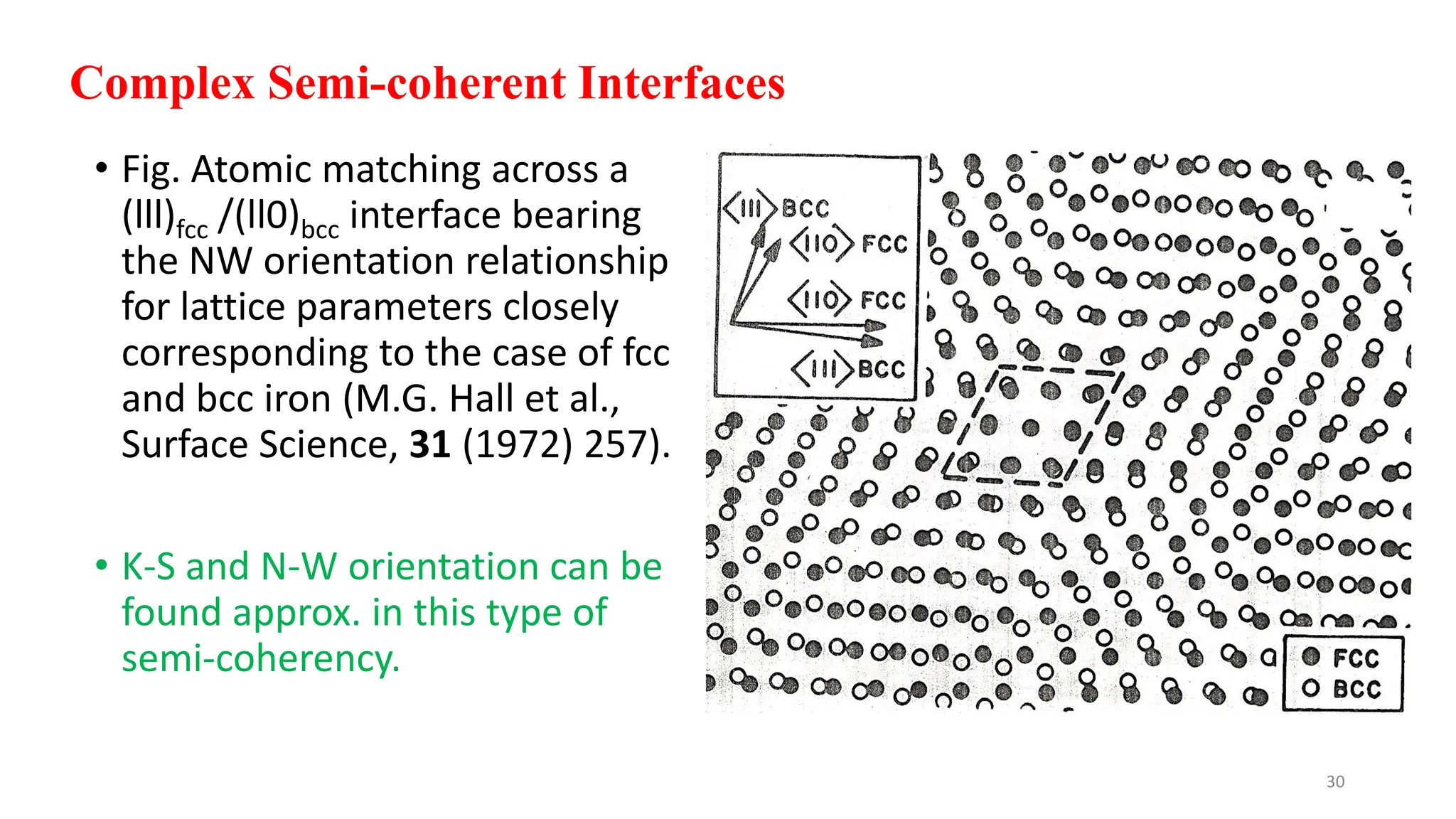
2. The interface between austenite and martensite, called the habit plane, must be semi-coherent to allow rapid transformation. It consists of a set of dislocations that provide continuity between the crystals.
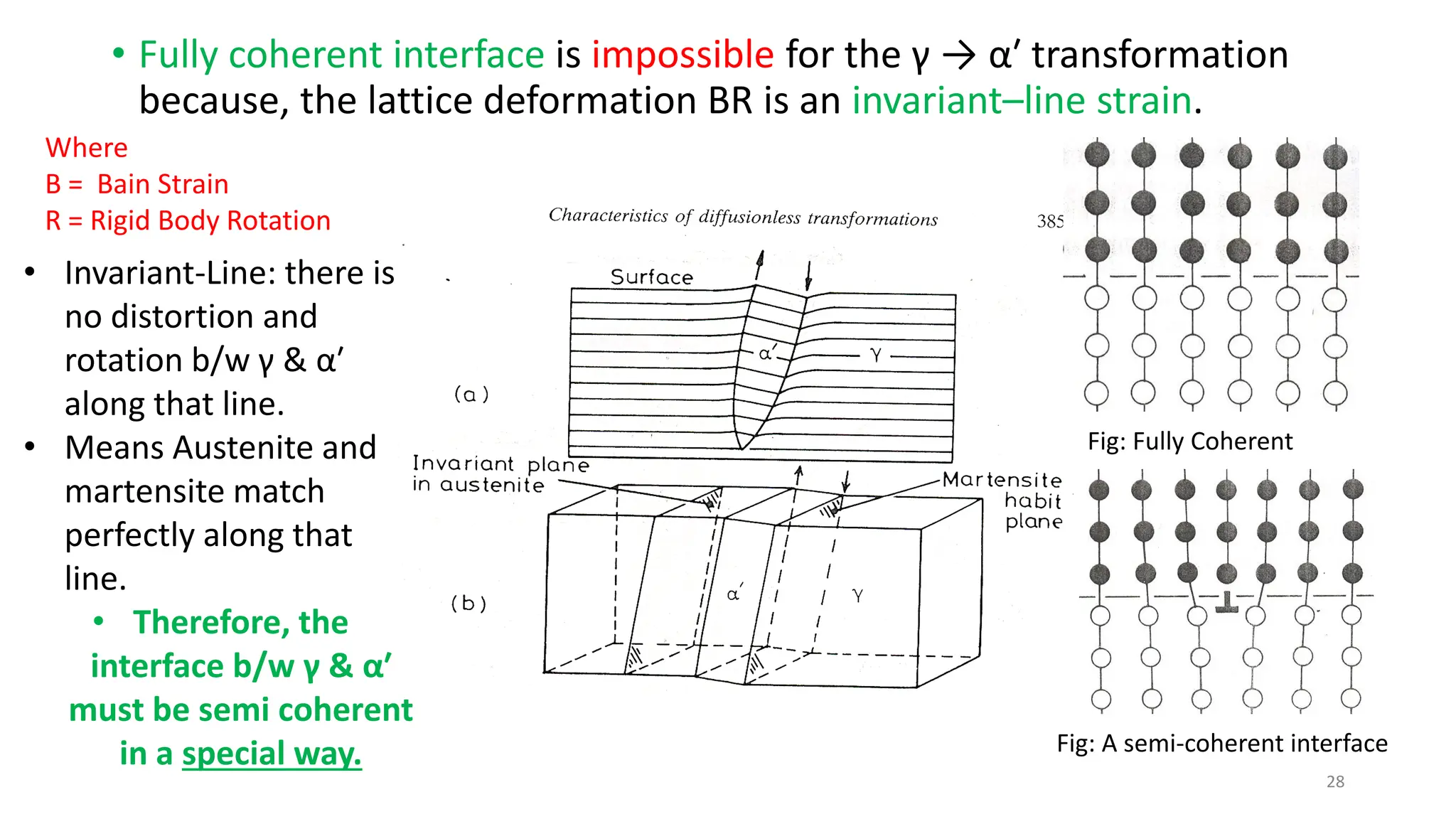
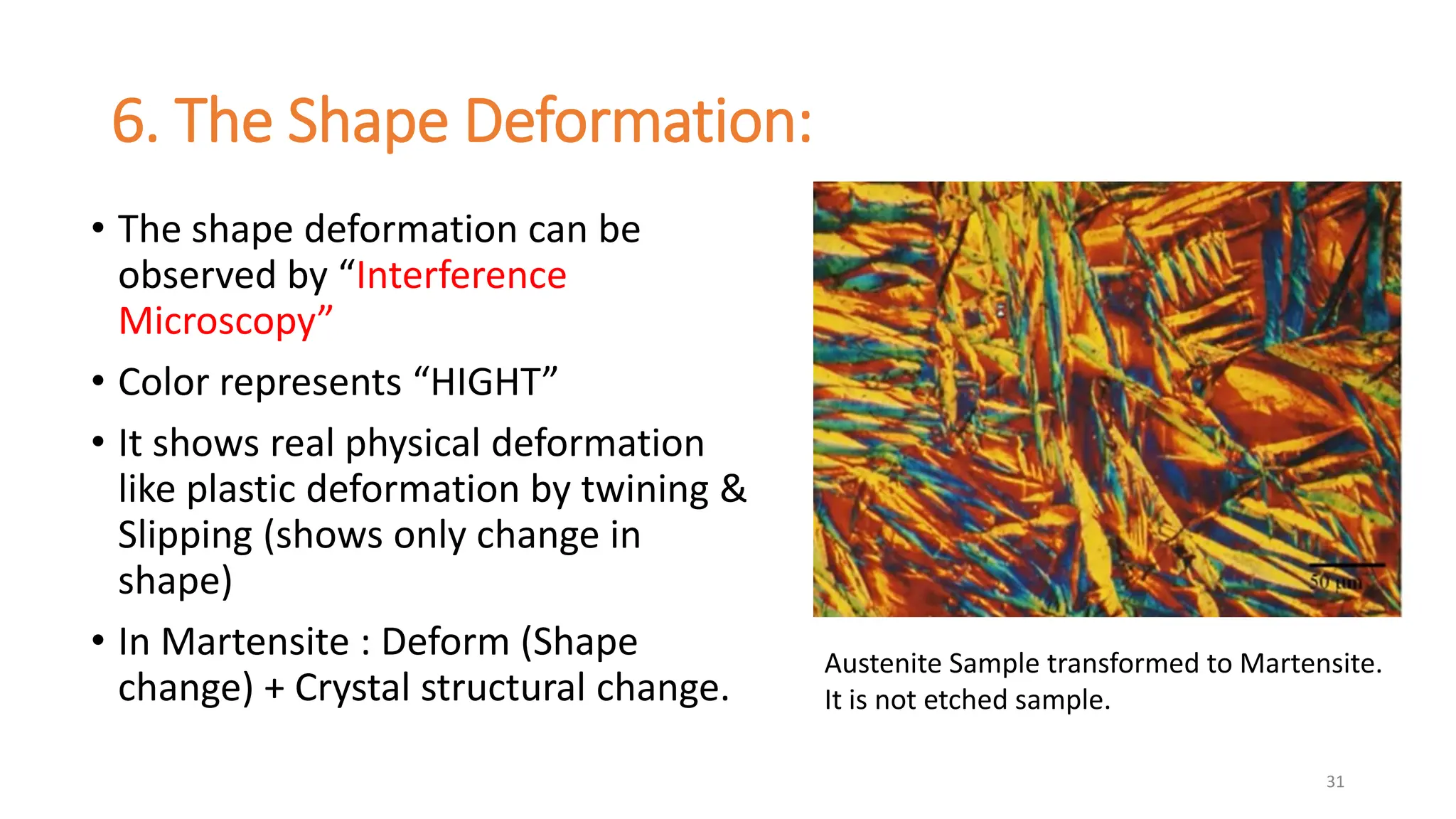
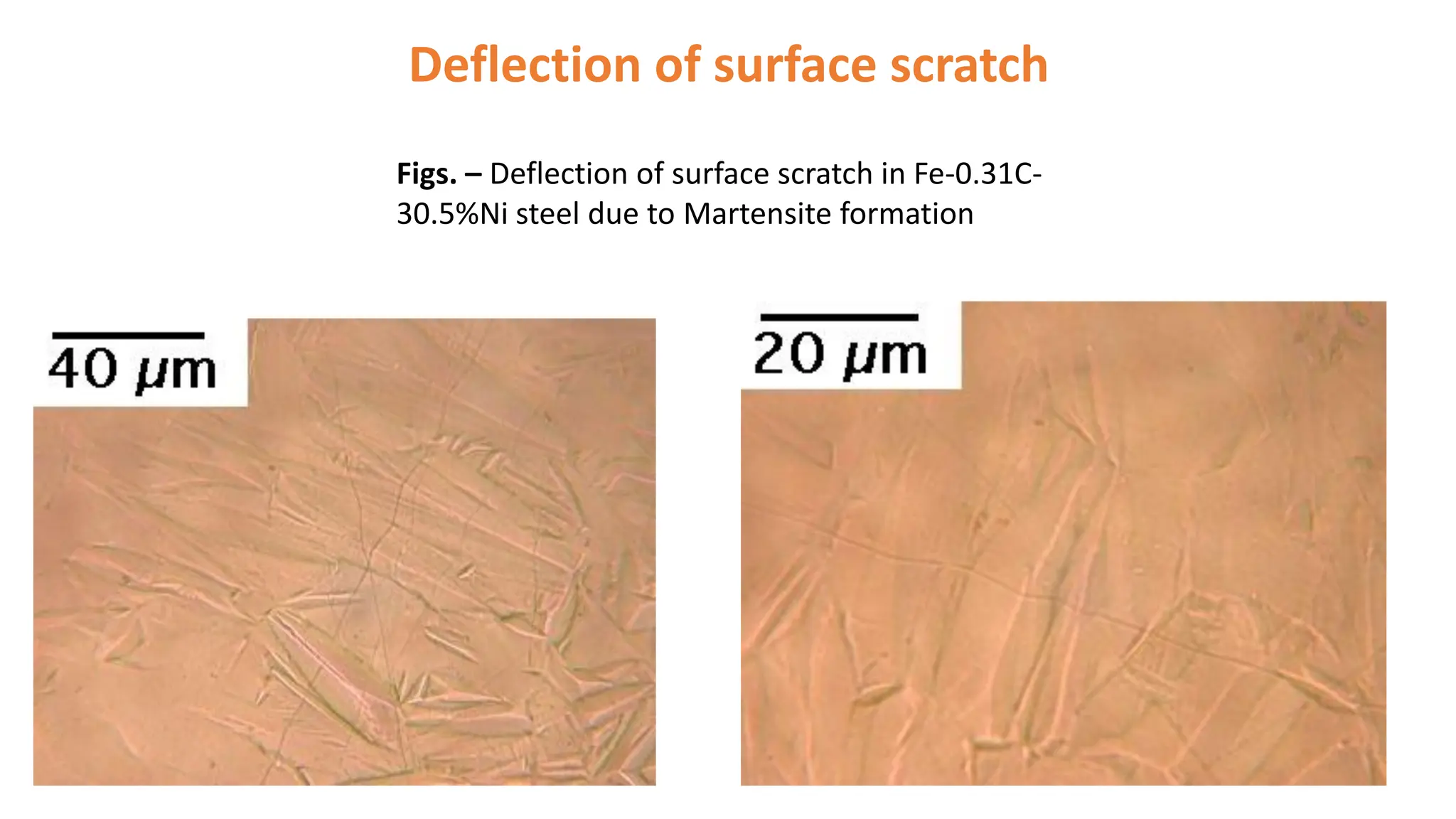
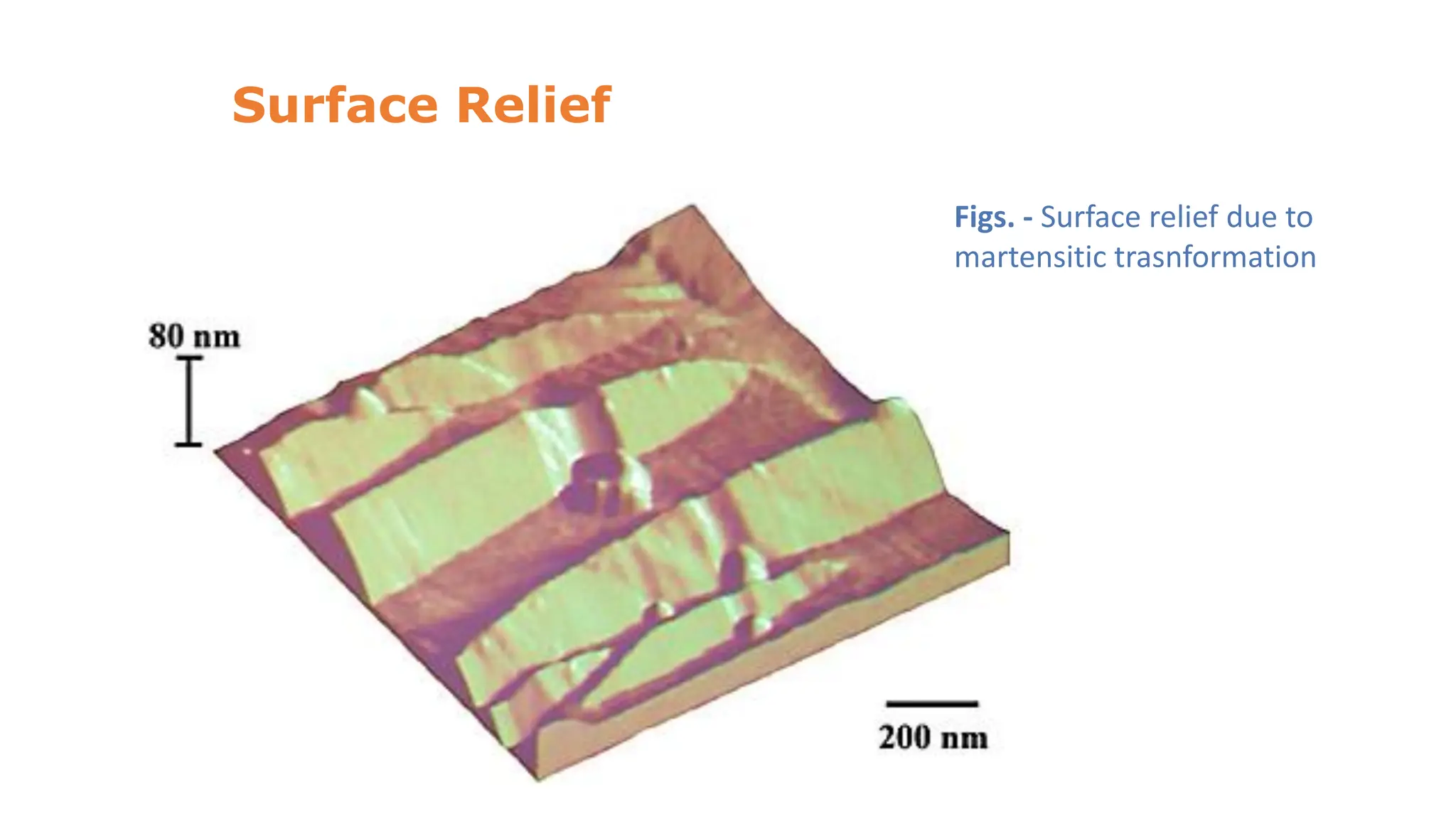
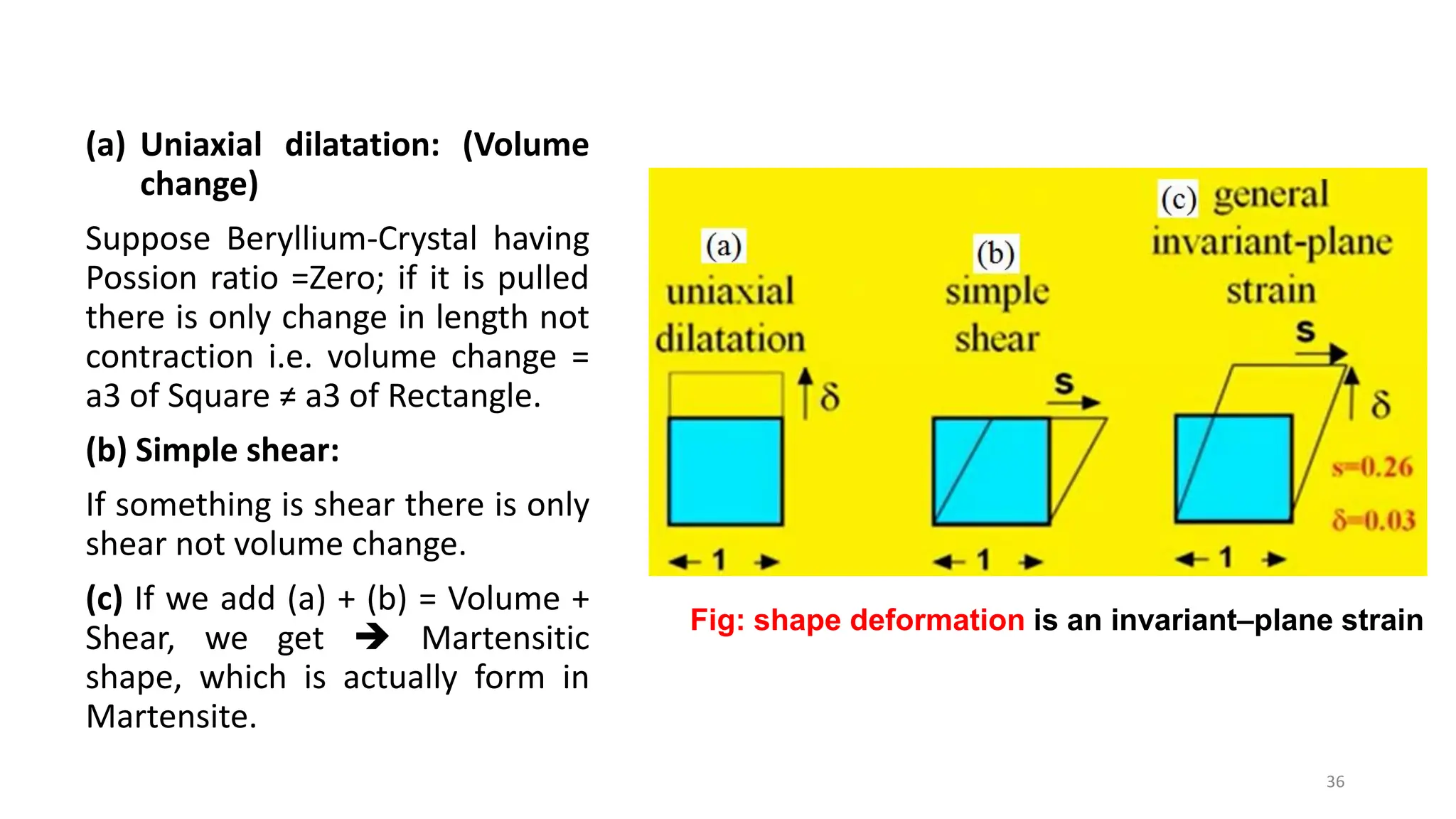
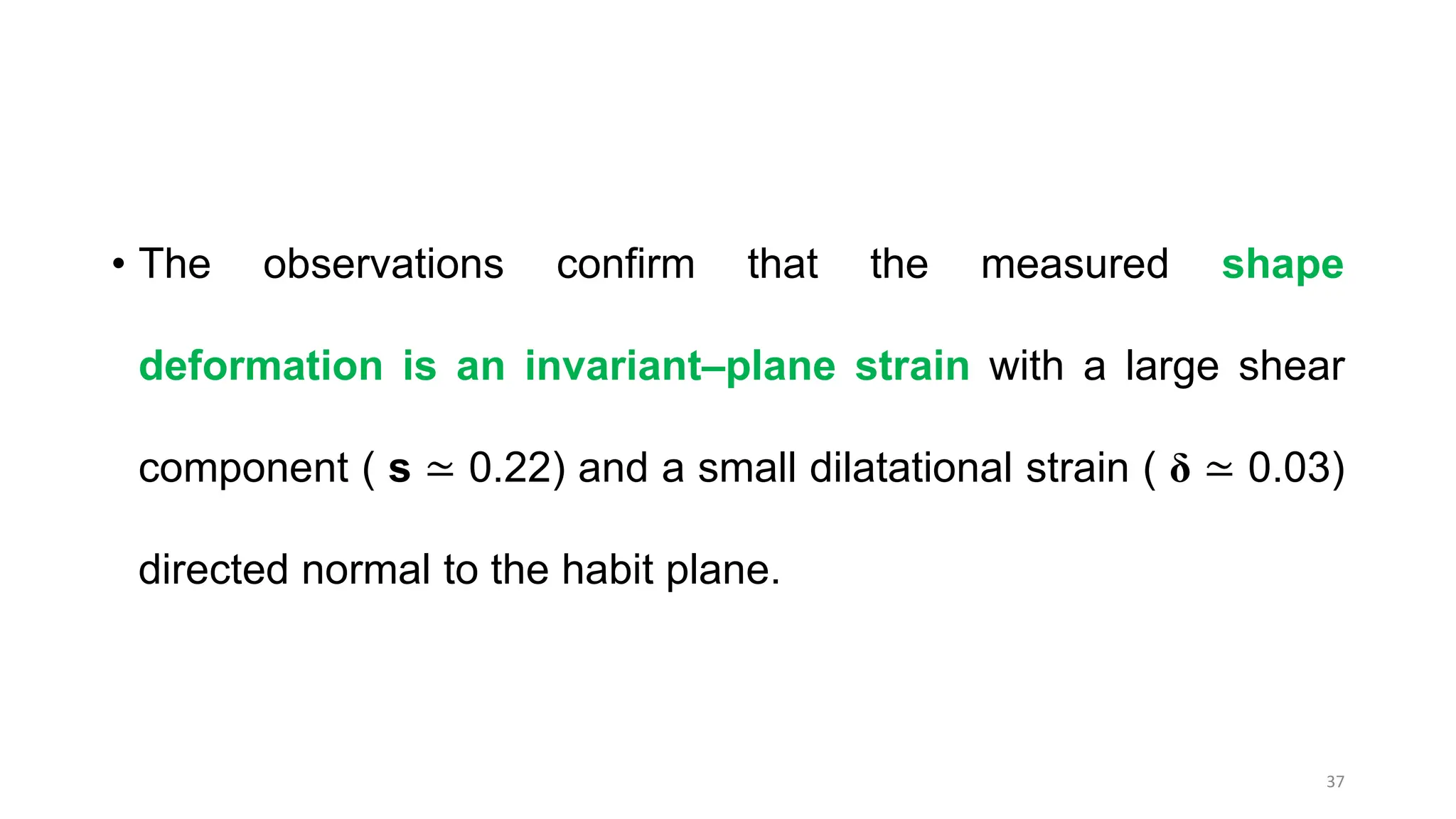
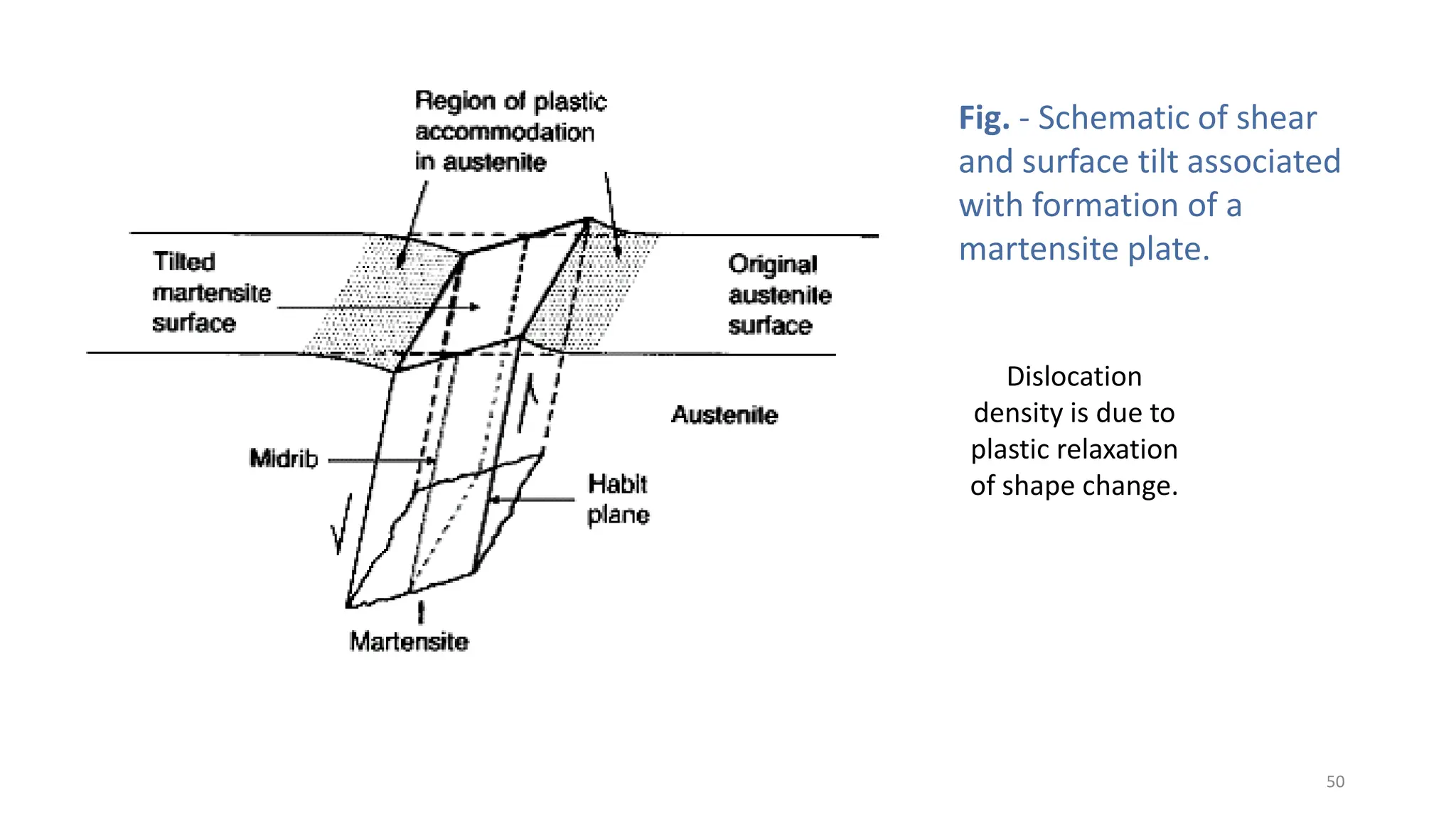
3. Martensitic transformation results in both a crystal structure change and a shape deformation of the material, as observed using interference microscopy techniques. The shape change is caused by a combination of volume expansion during the structure change coupled with simple shear deformation.

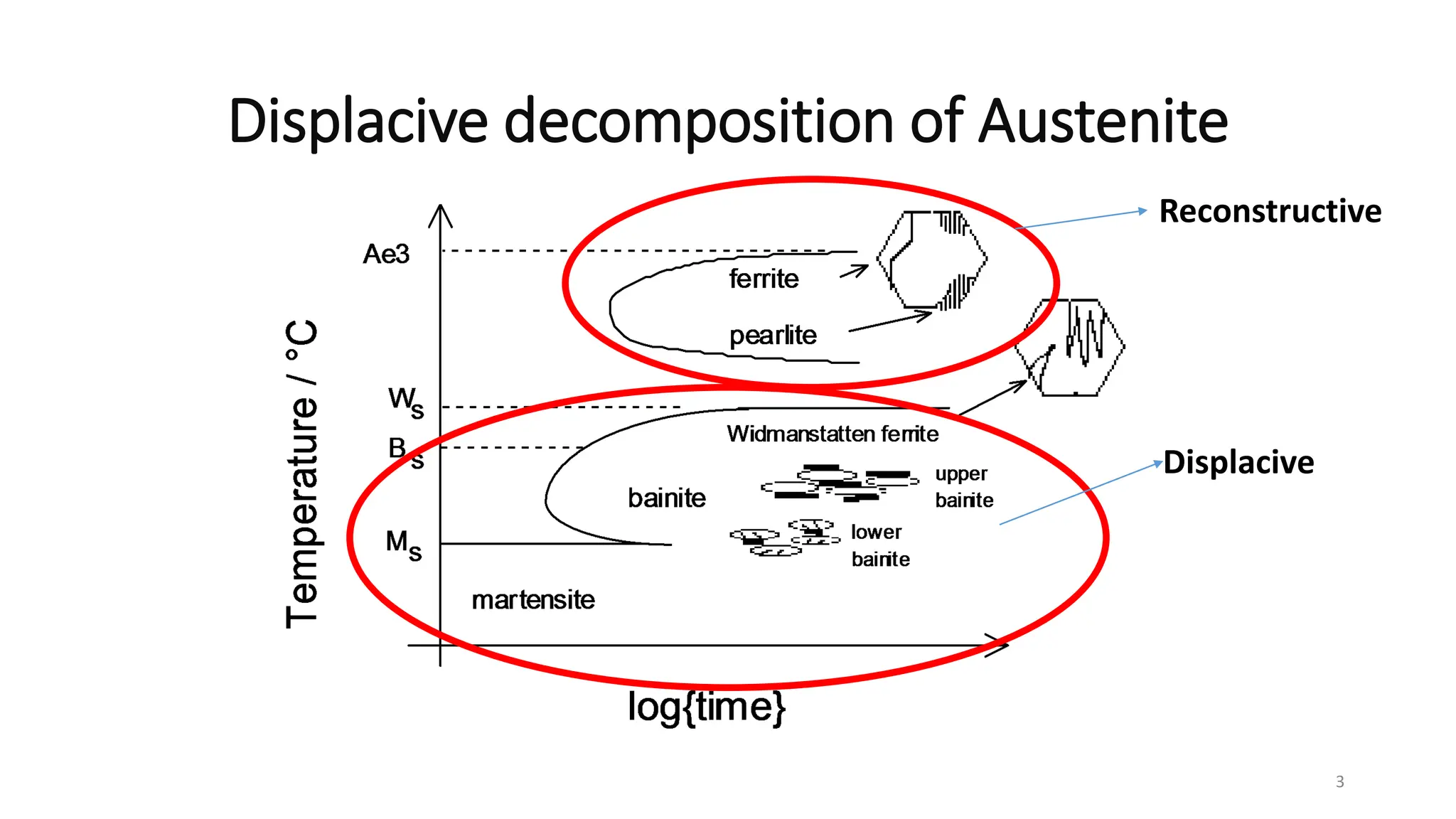
![•The “displacive transformation products” are very
important form engineering point of view, because
the volume fraction of these products directly affects
the mechanical properties of the steels.
•For example steels in bainite conditions show a
remarkable combination of strengthand toughness
[Nakasugi et al. 1983]
•and the large volume fraction of acicular ferrite
enhances the toughness [Garland and Kirkwood,
1975].
4](https://image.slidesharecdn.com/lectureseries-ddisplacivetransformationmartensite-240305115600-e6a11b54/75/Phase-Transformation-in-Steel-Lecture-Series-D-Displacive-Transformation-Martensite-pdf-4-2048.jpg)