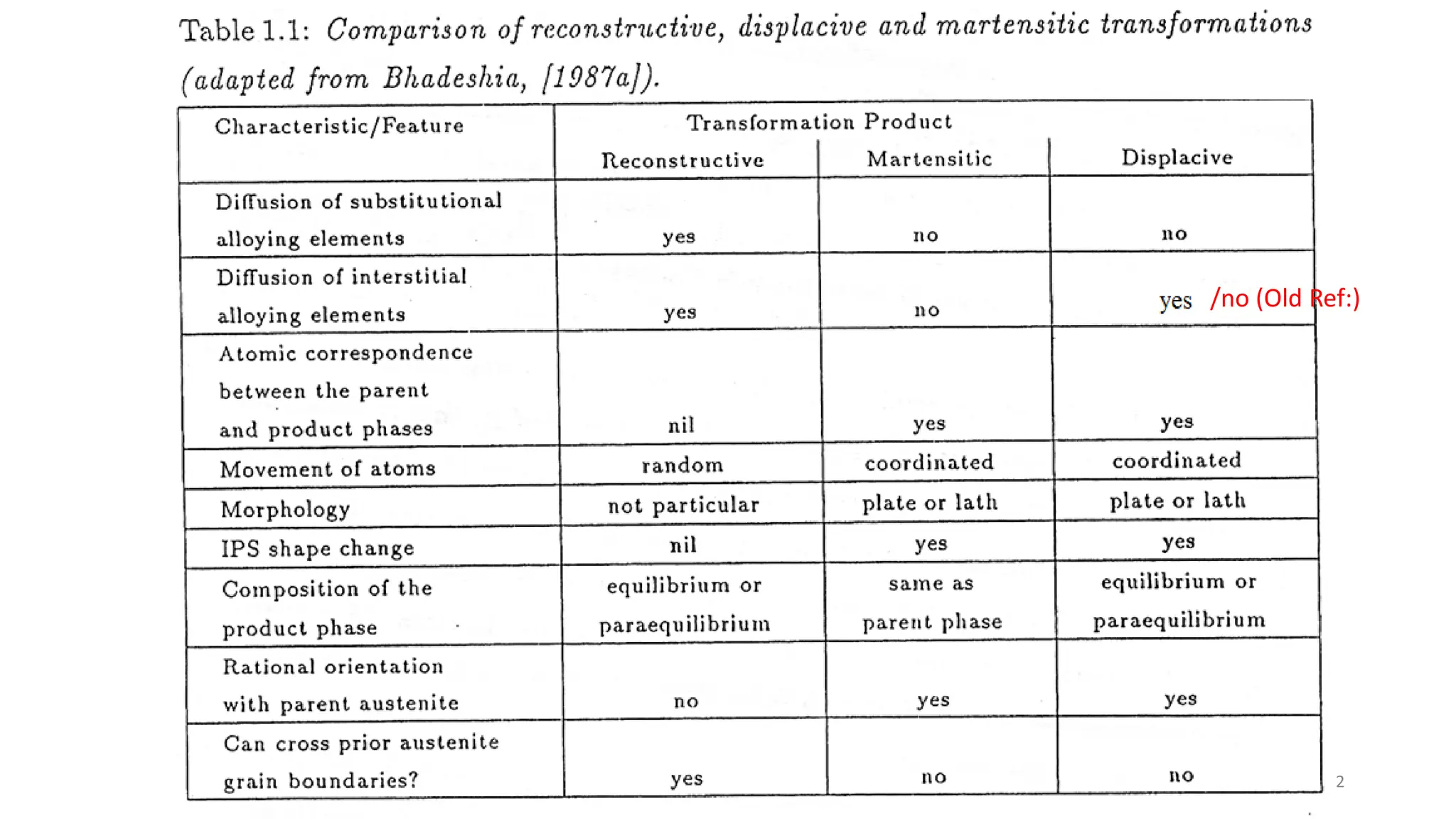
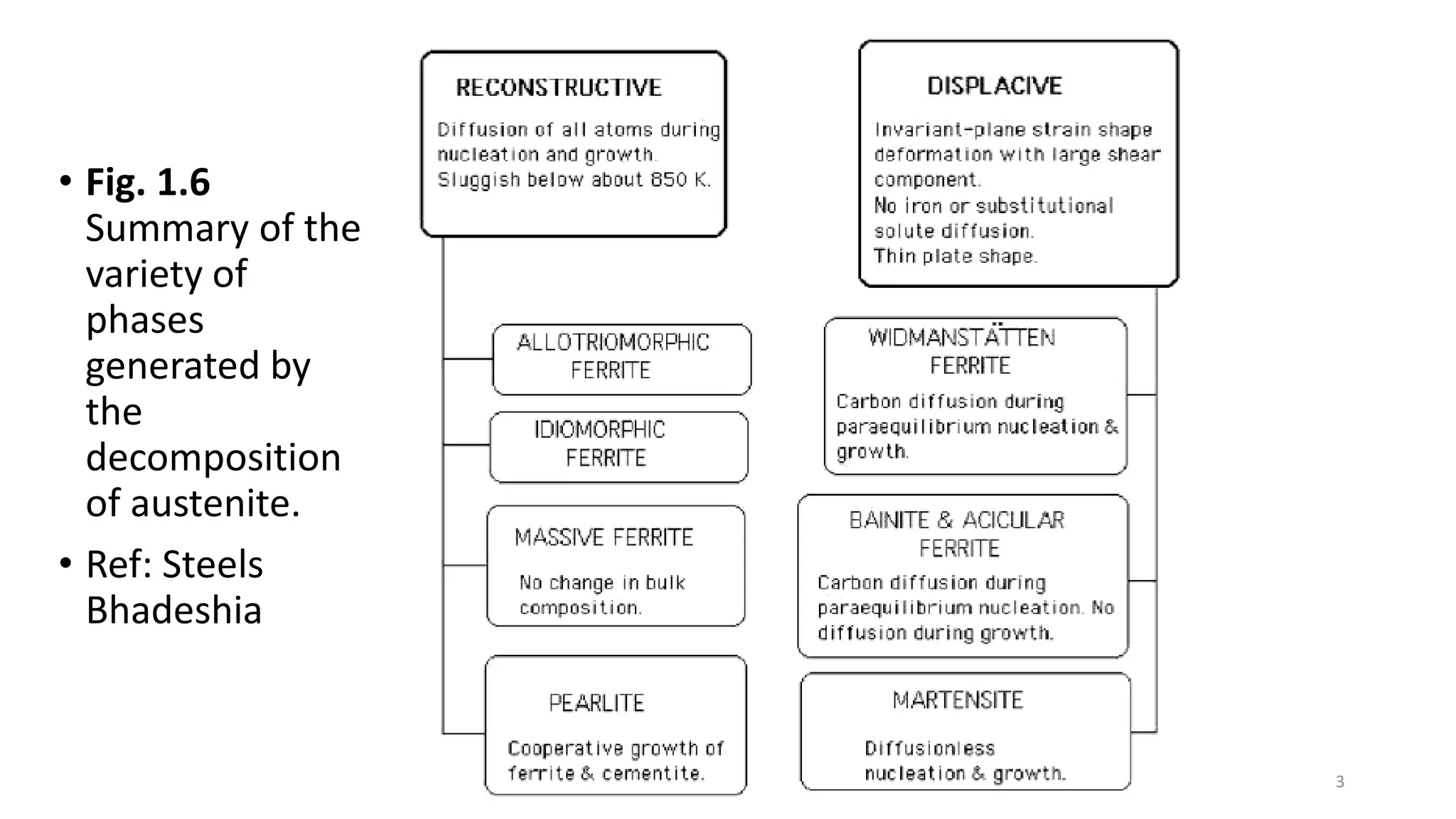
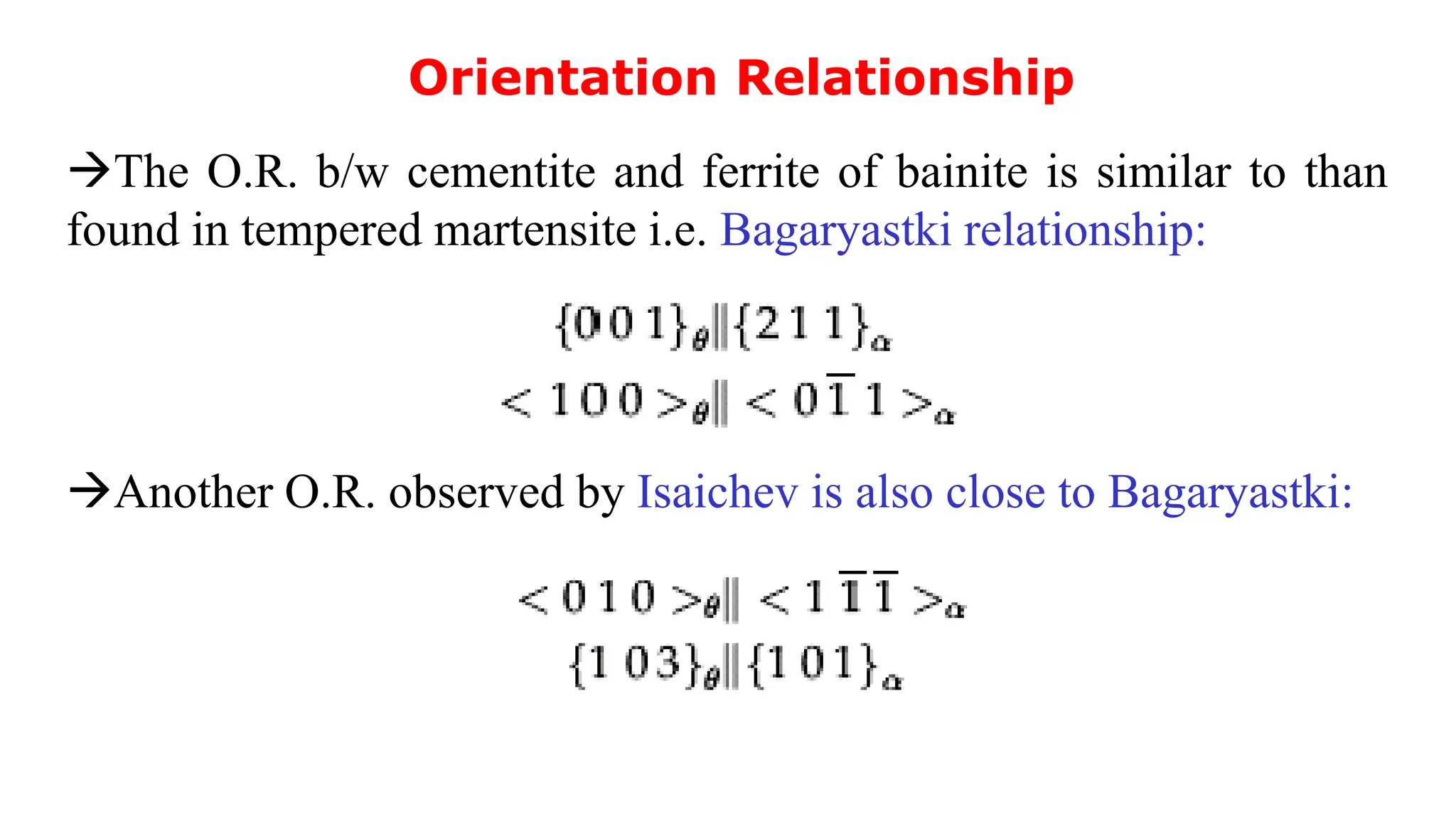
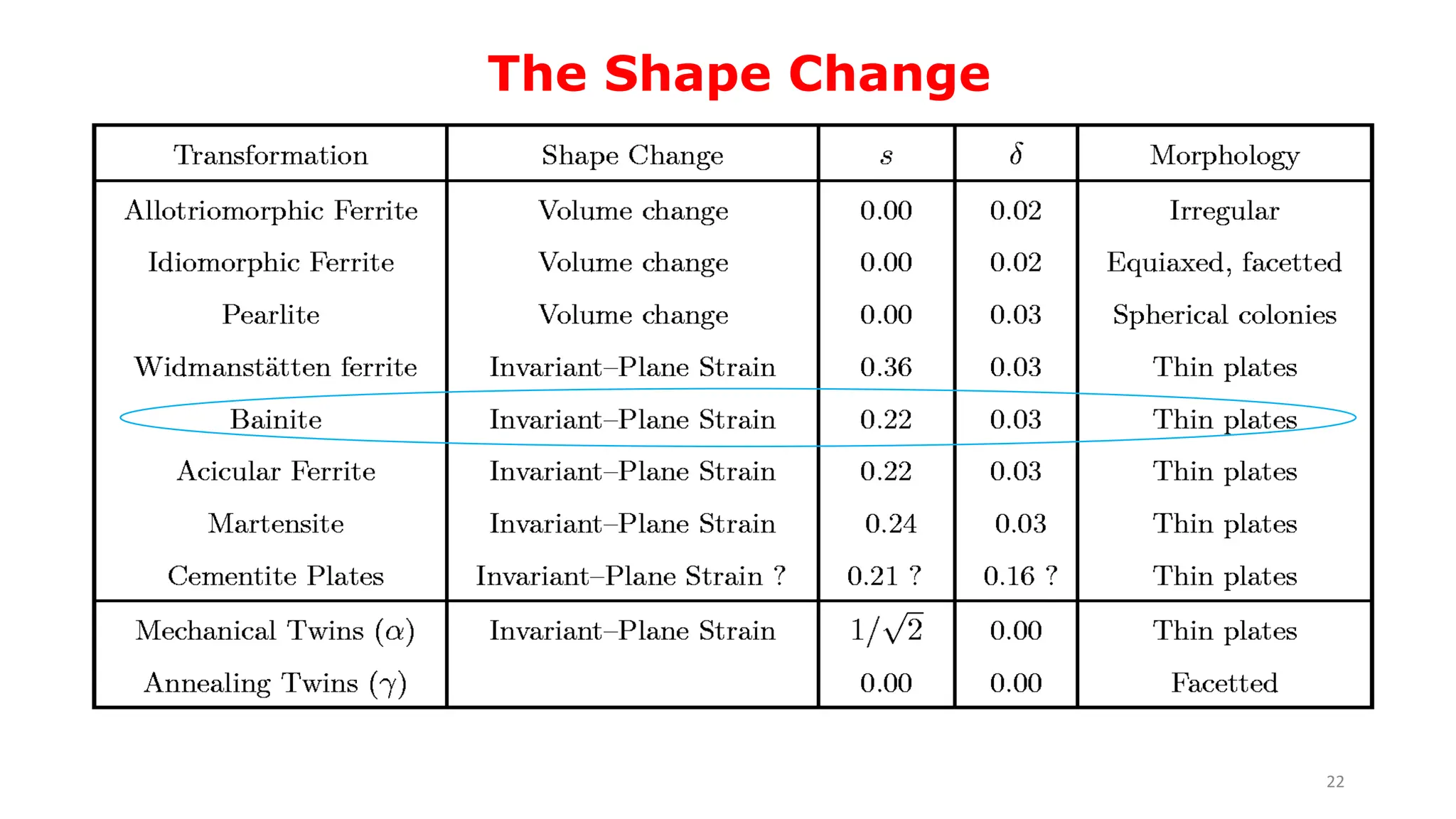
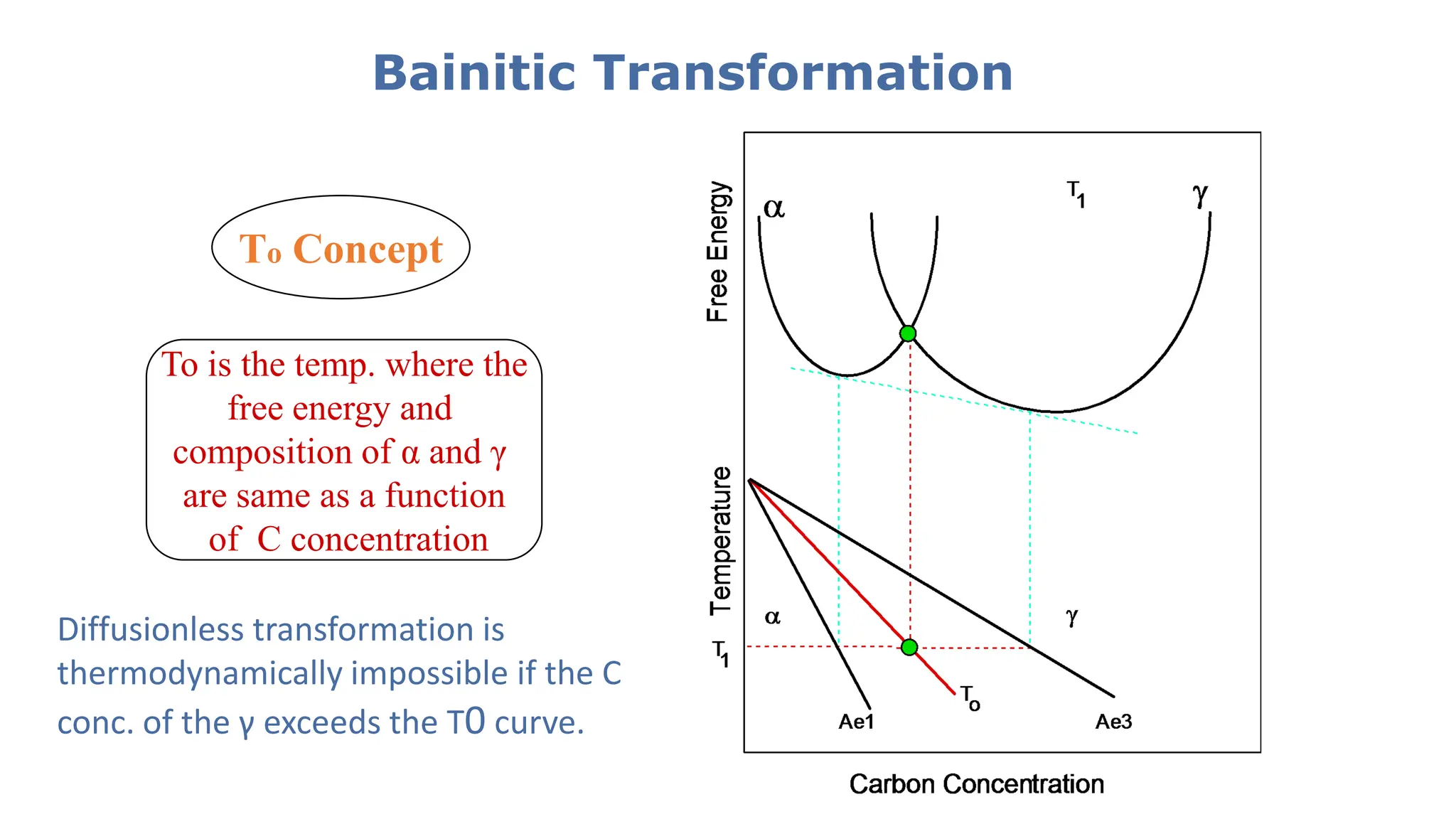
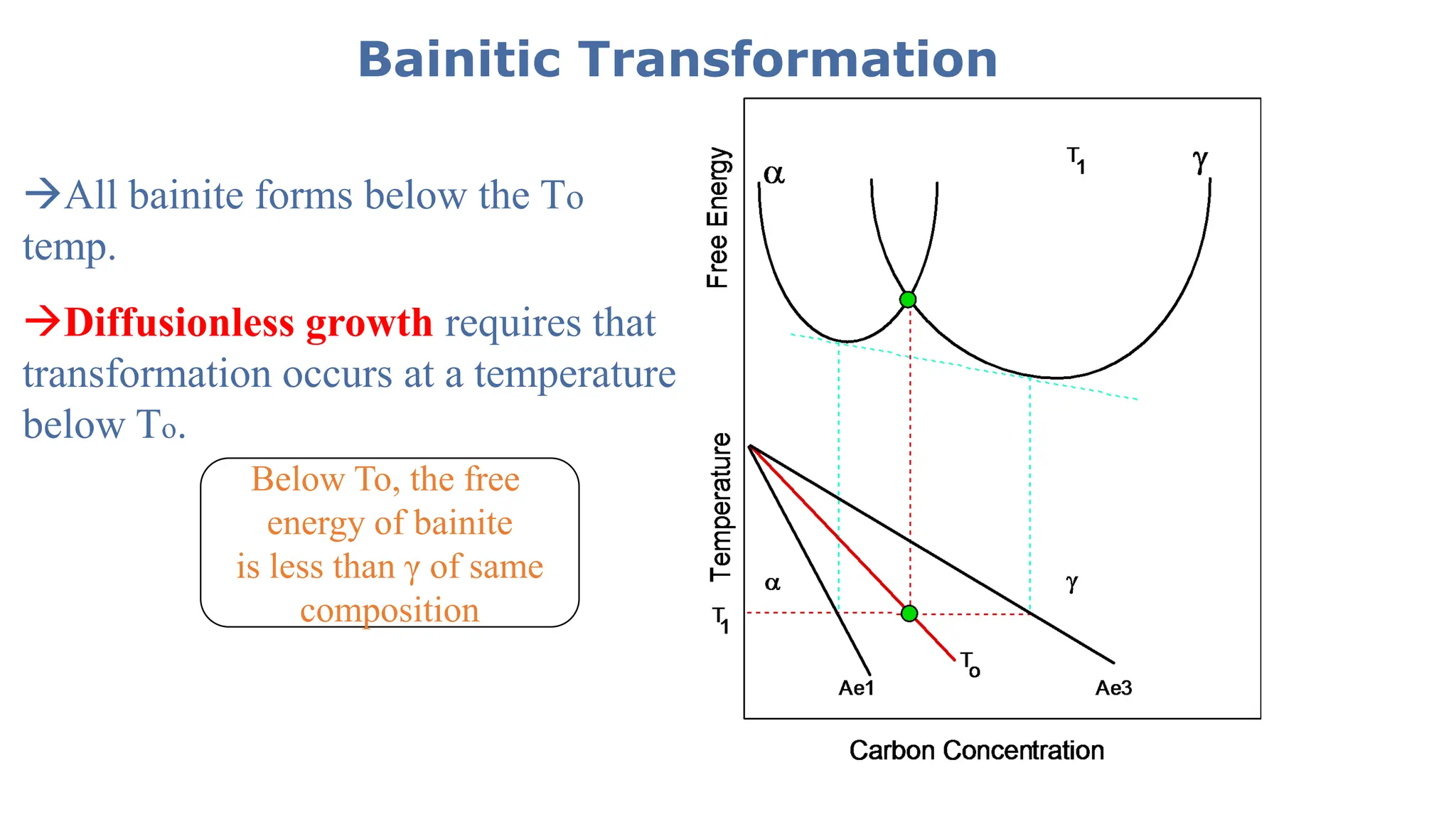
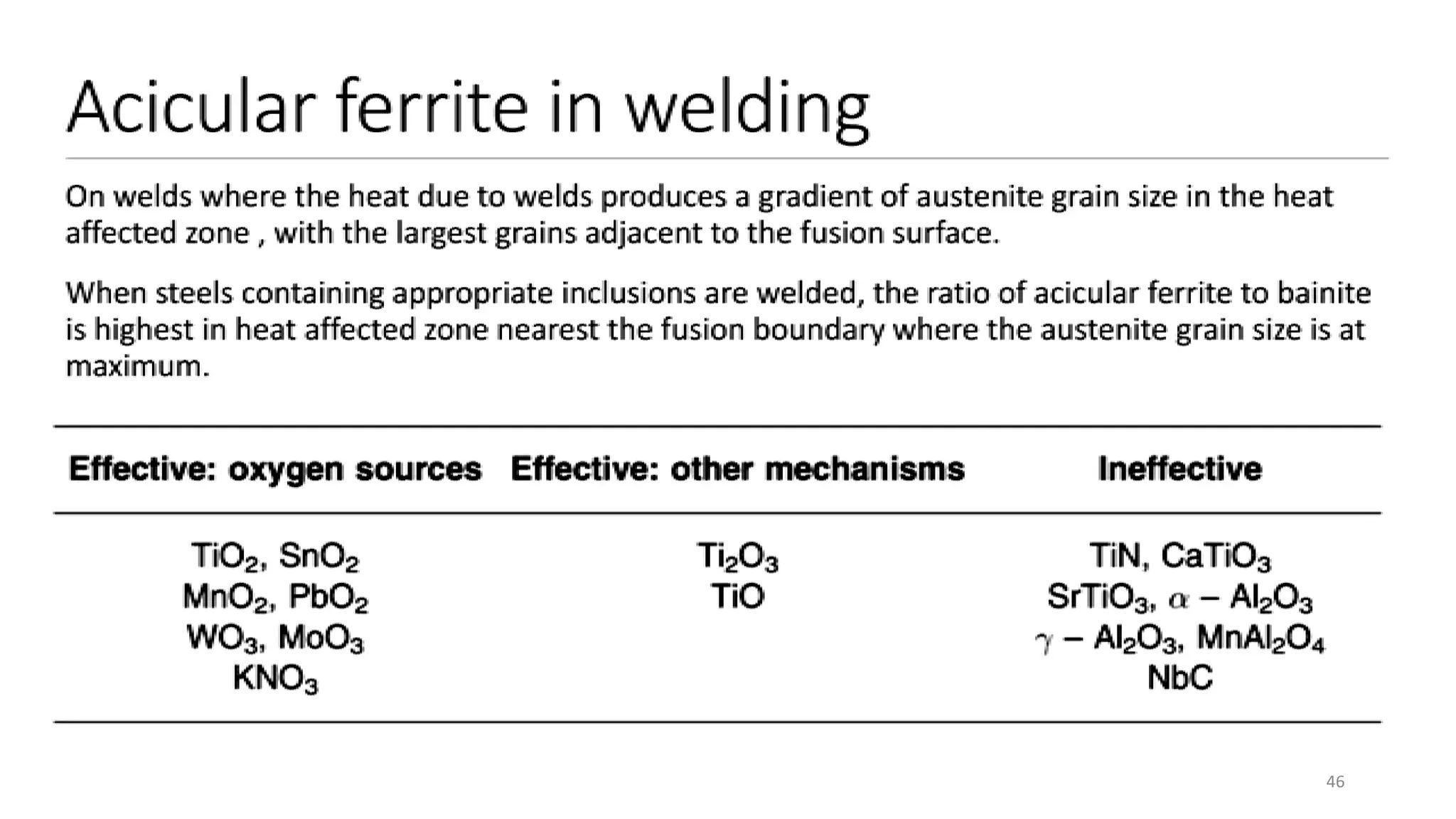
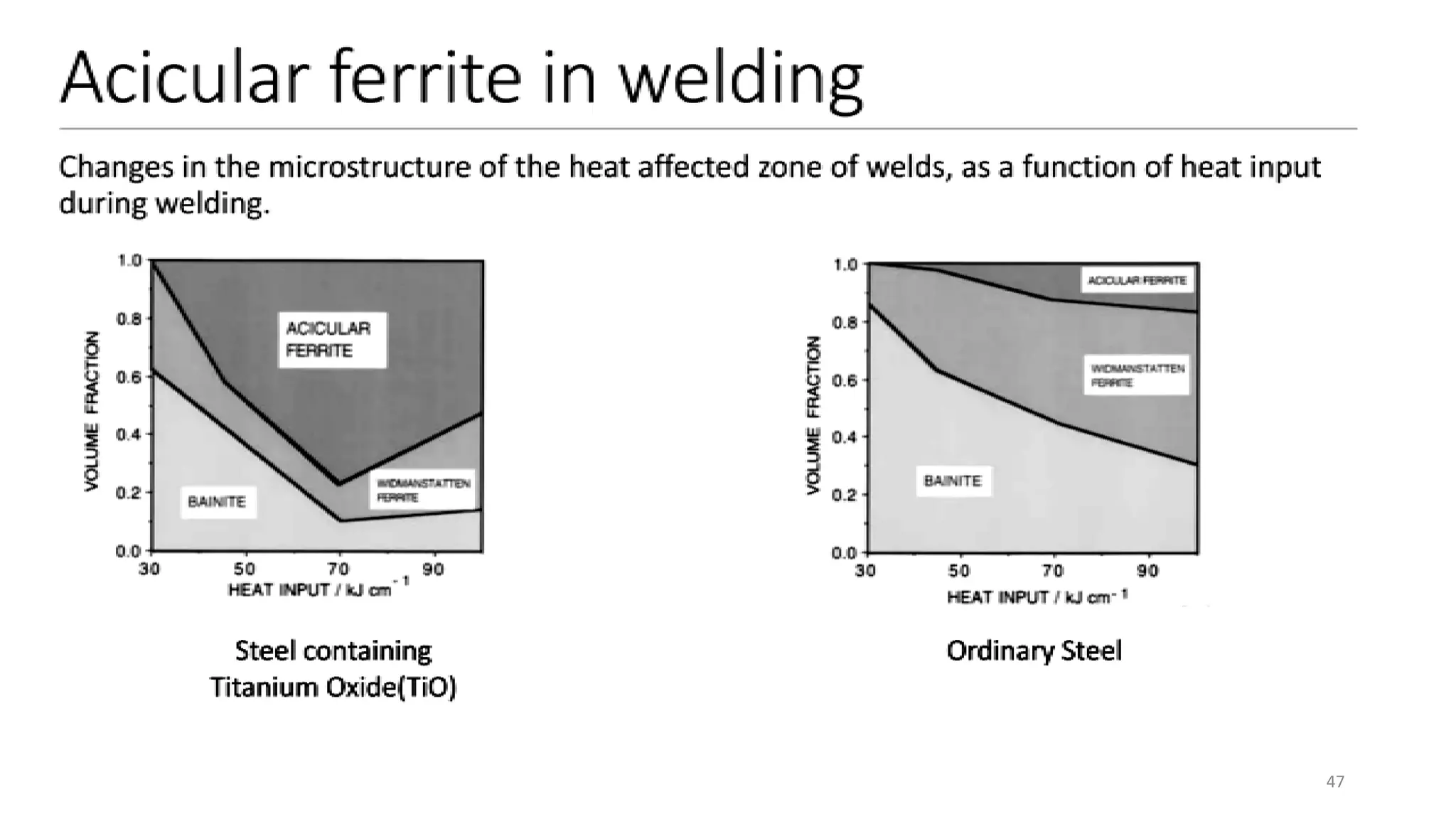
1) Phase transformations in solid metals include the decomposition of austenite into other phases such as bainite, Widmanstätten ferrite, and acicular ferrite.
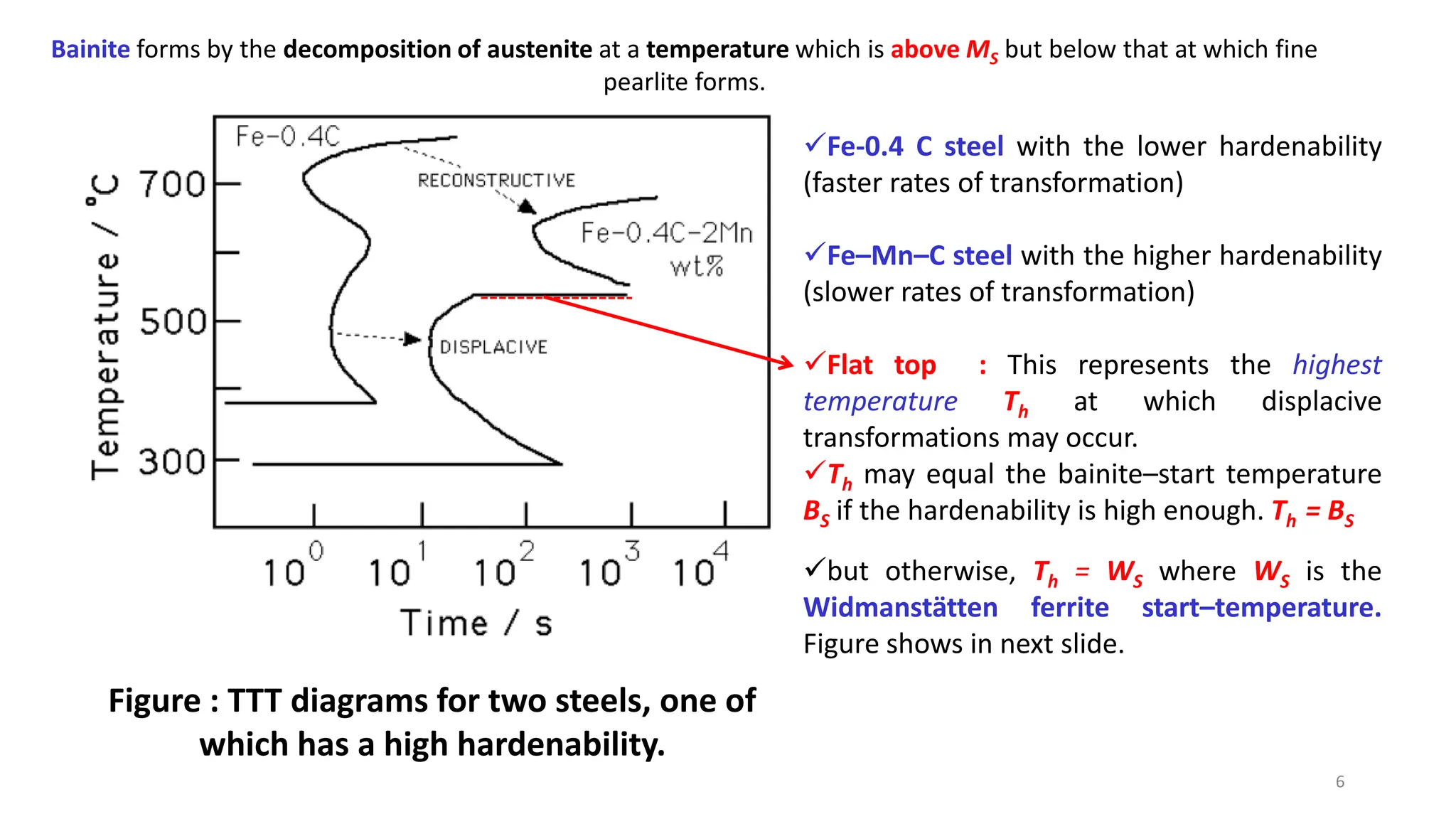
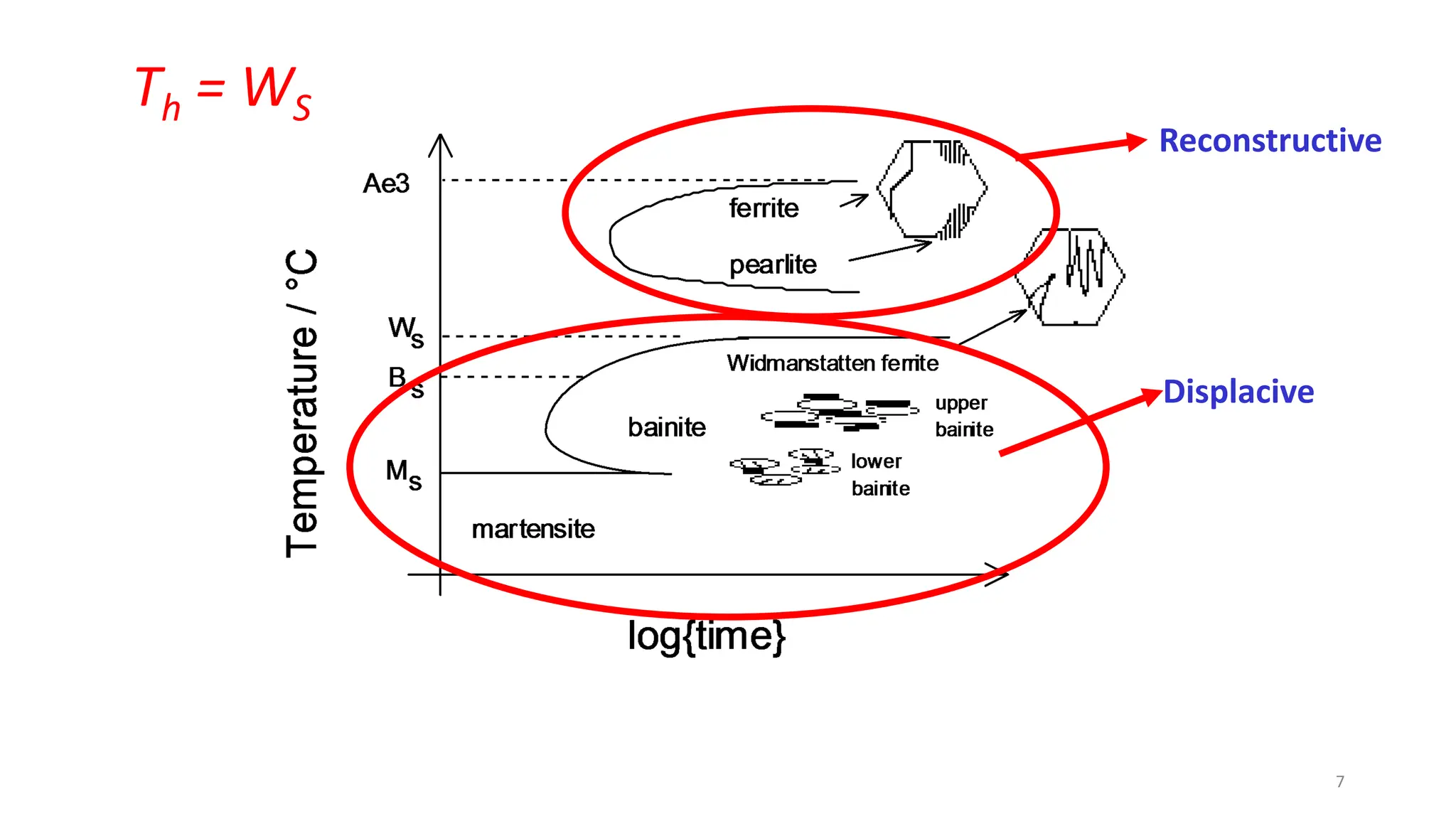
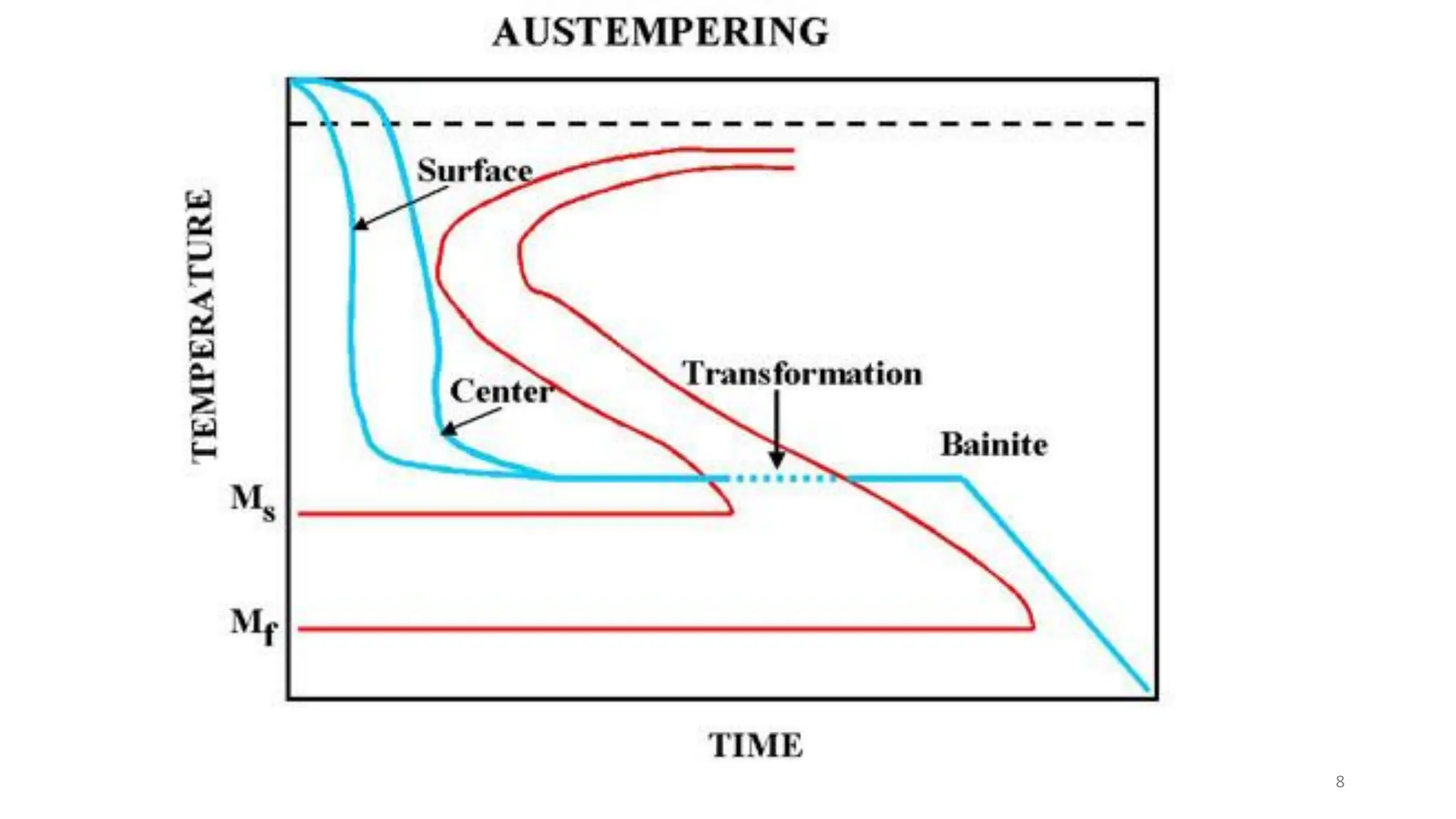
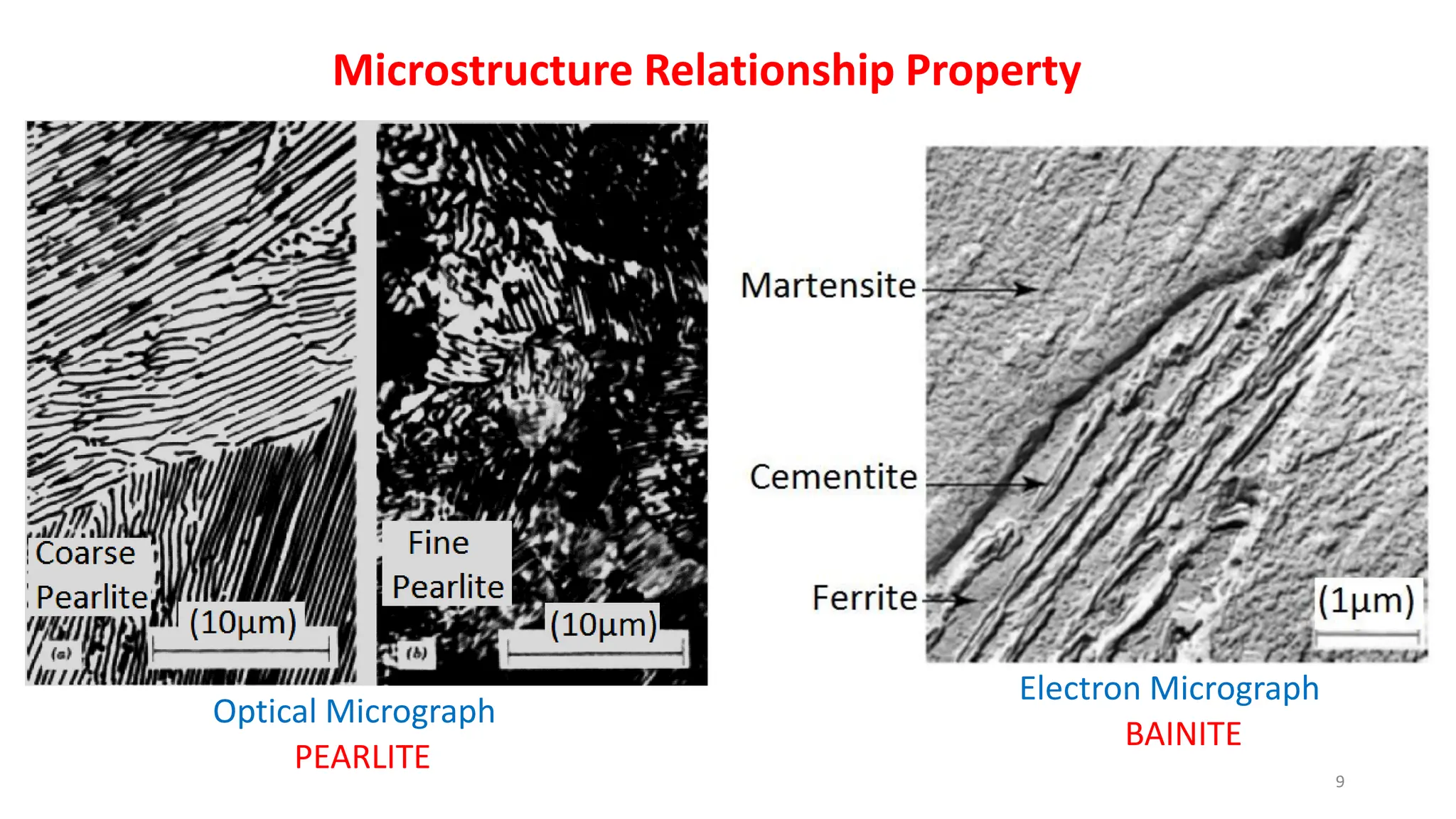
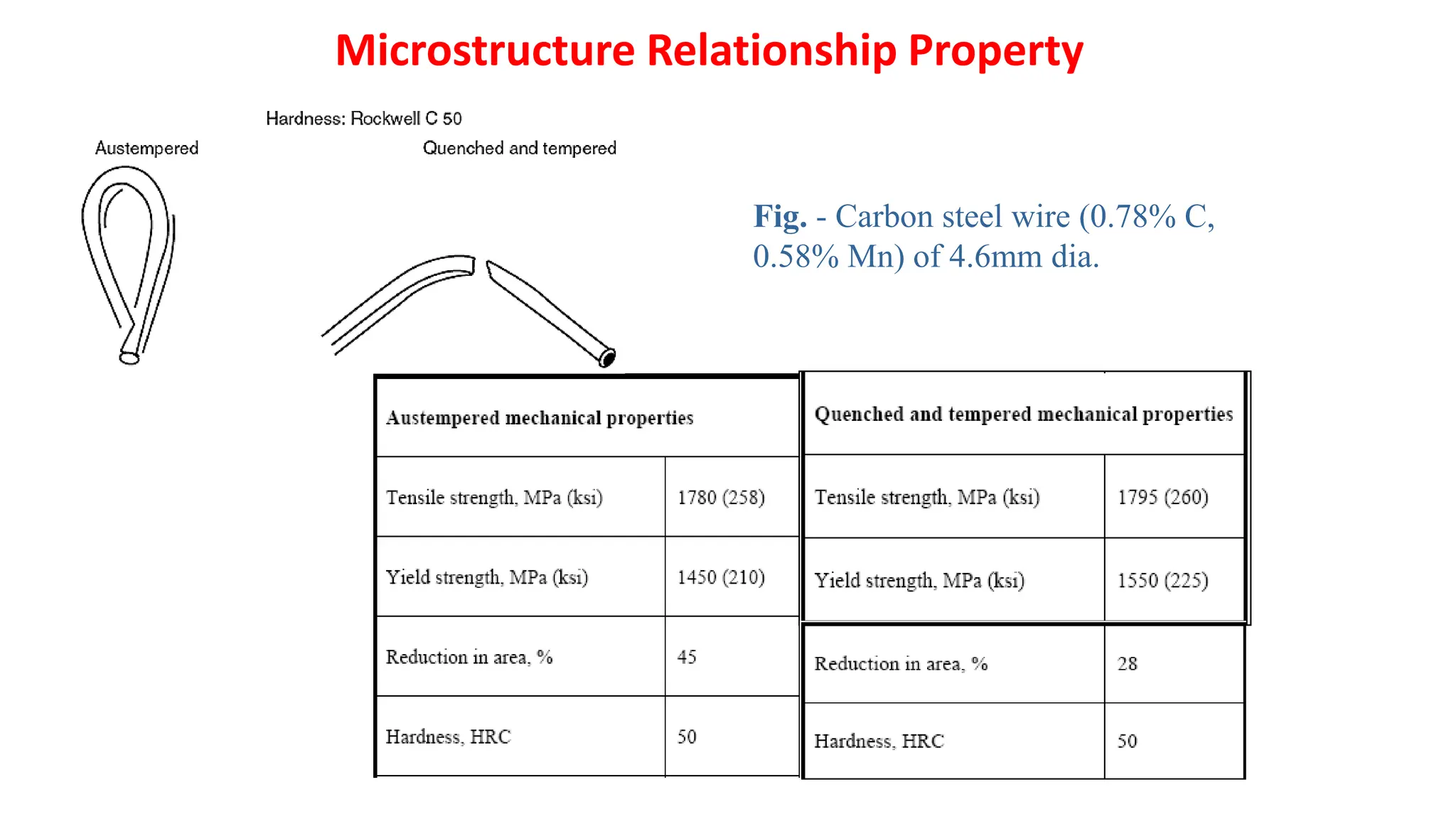
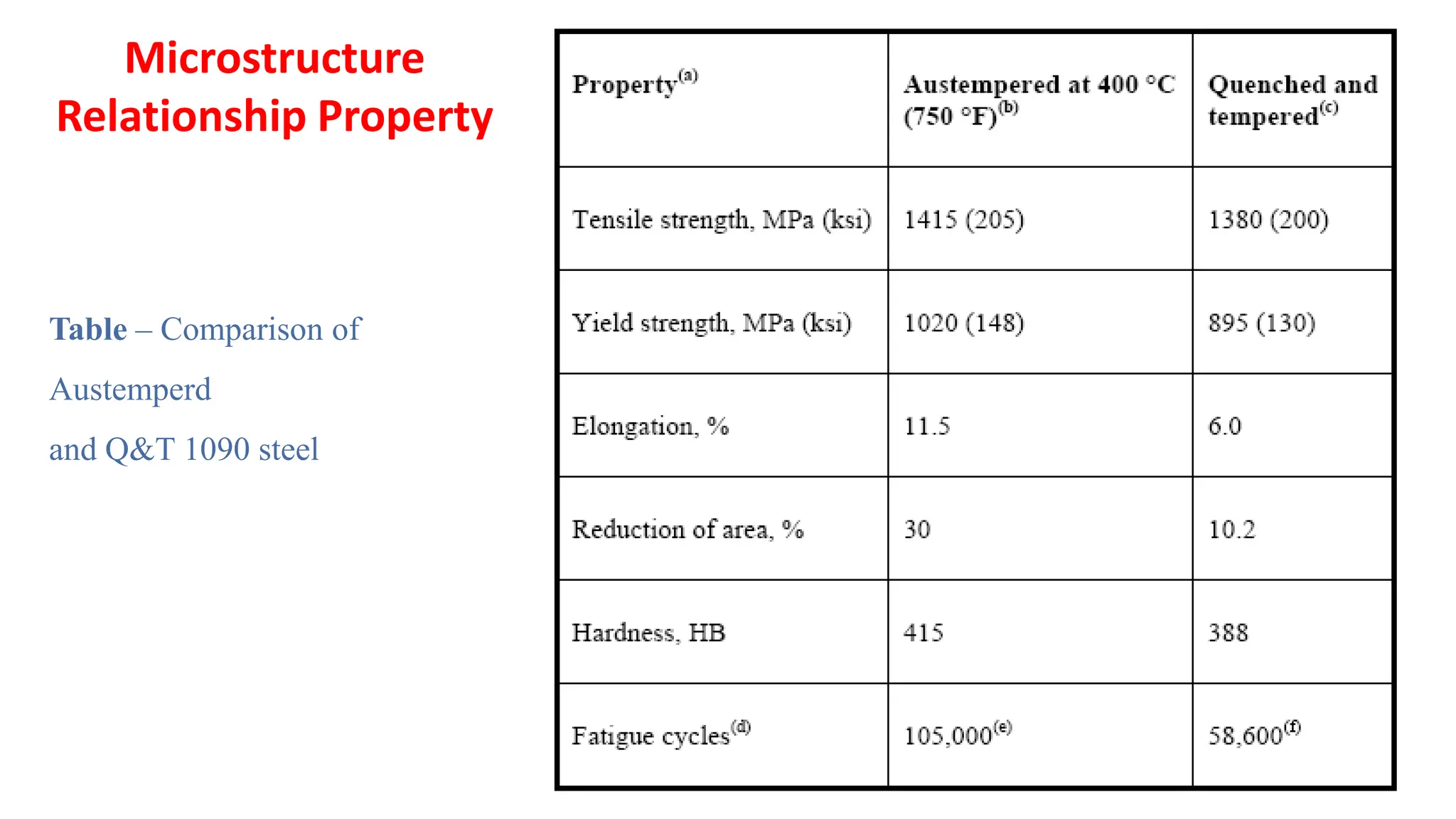
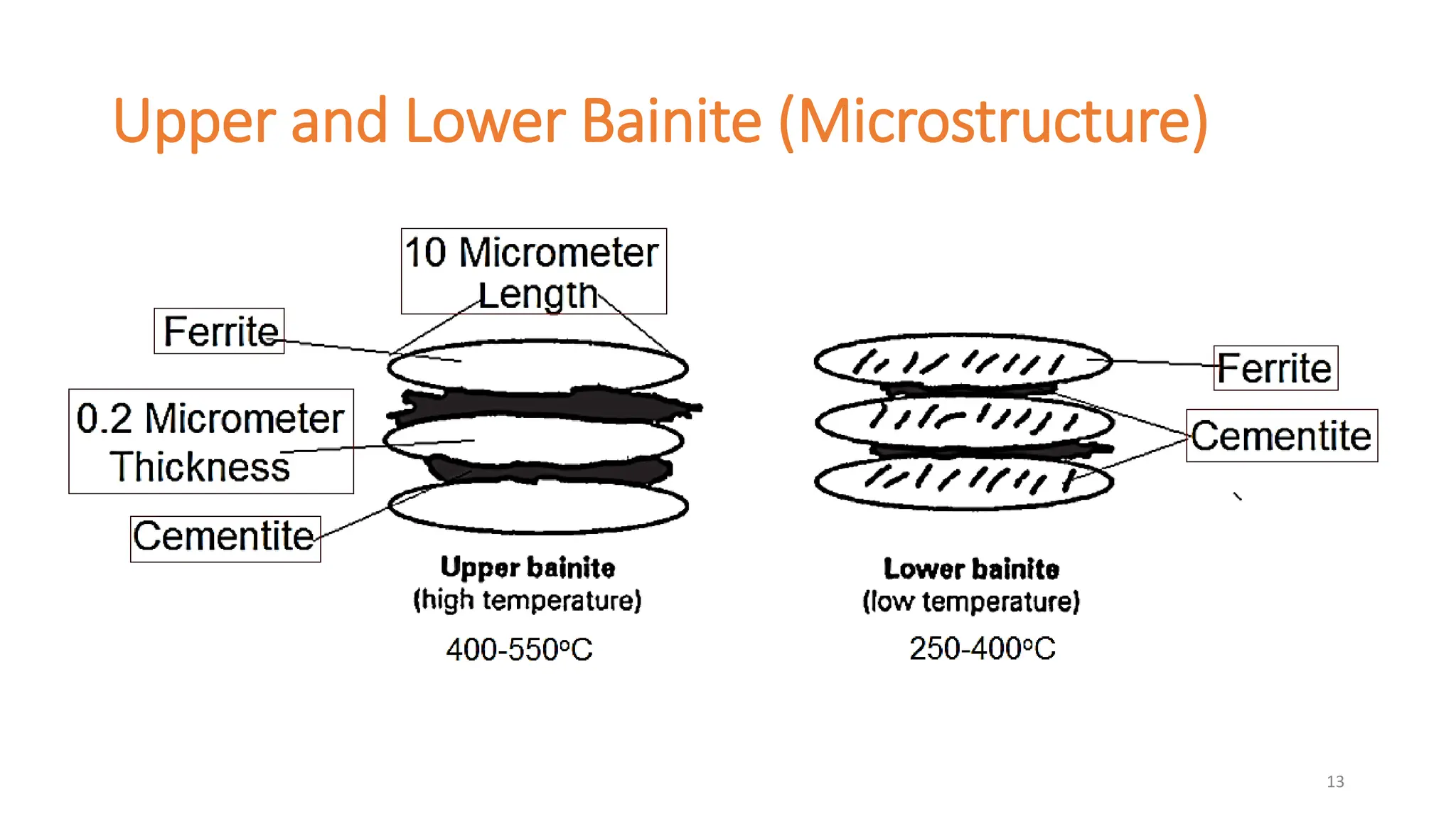
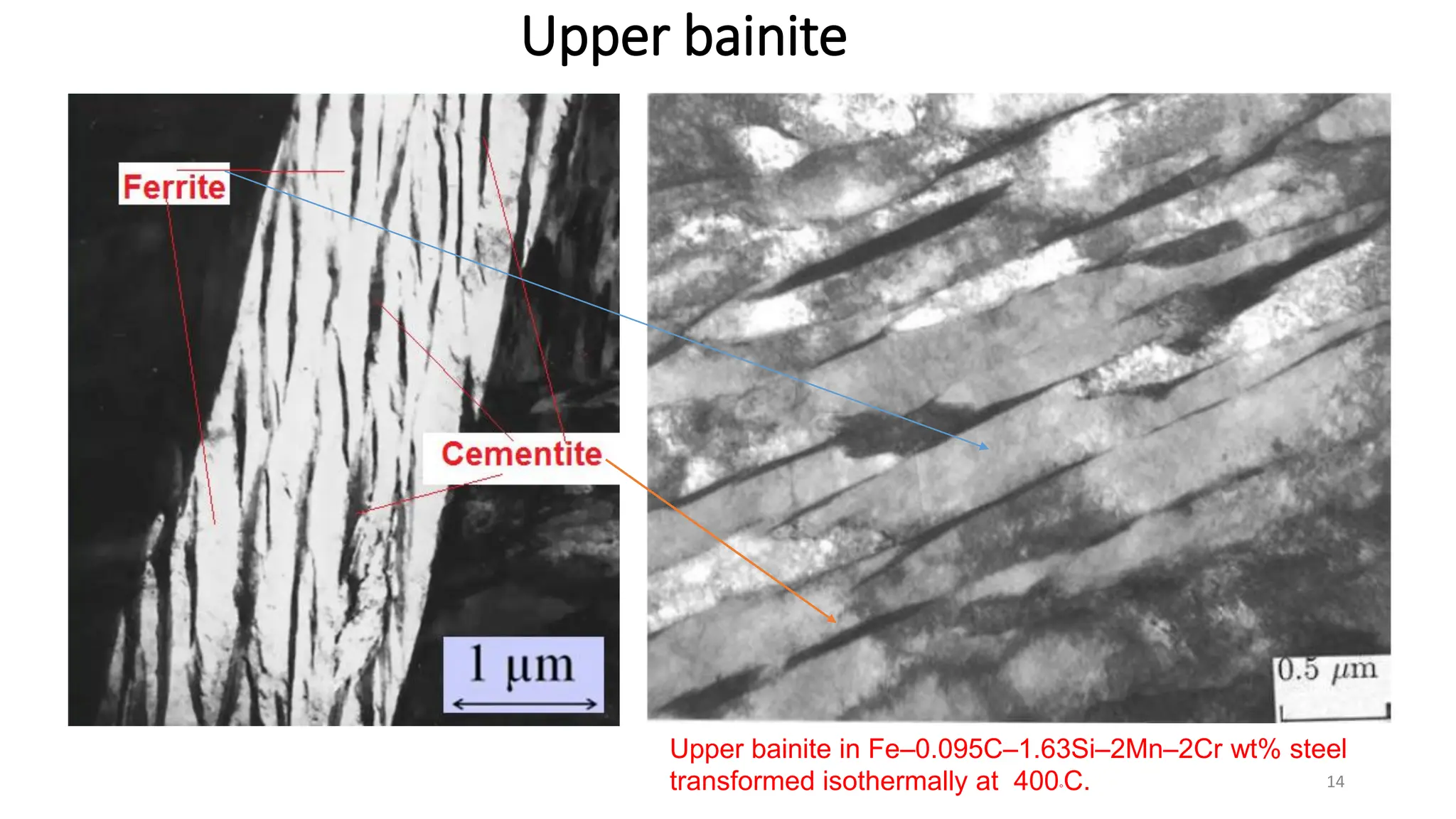
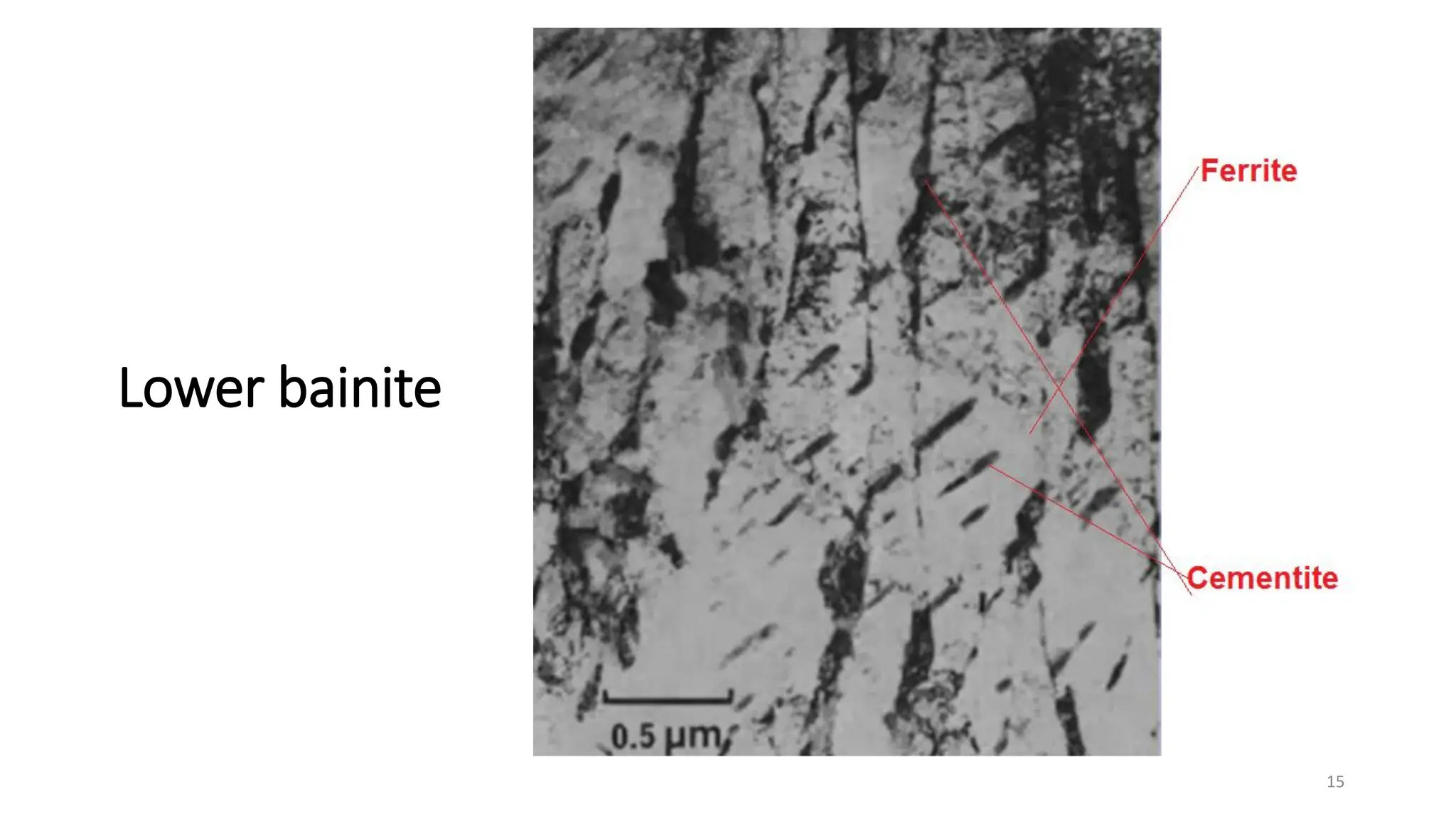
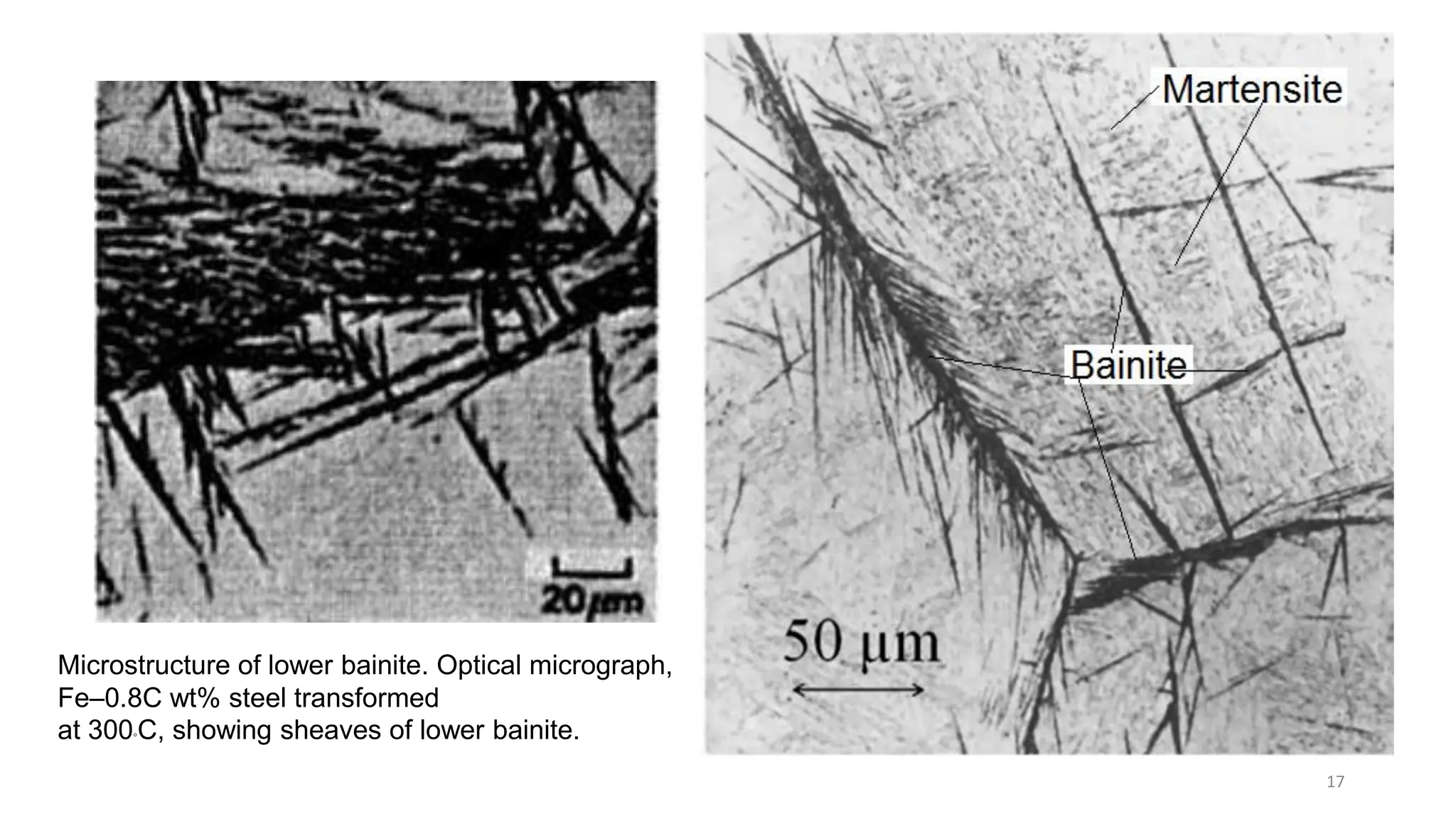
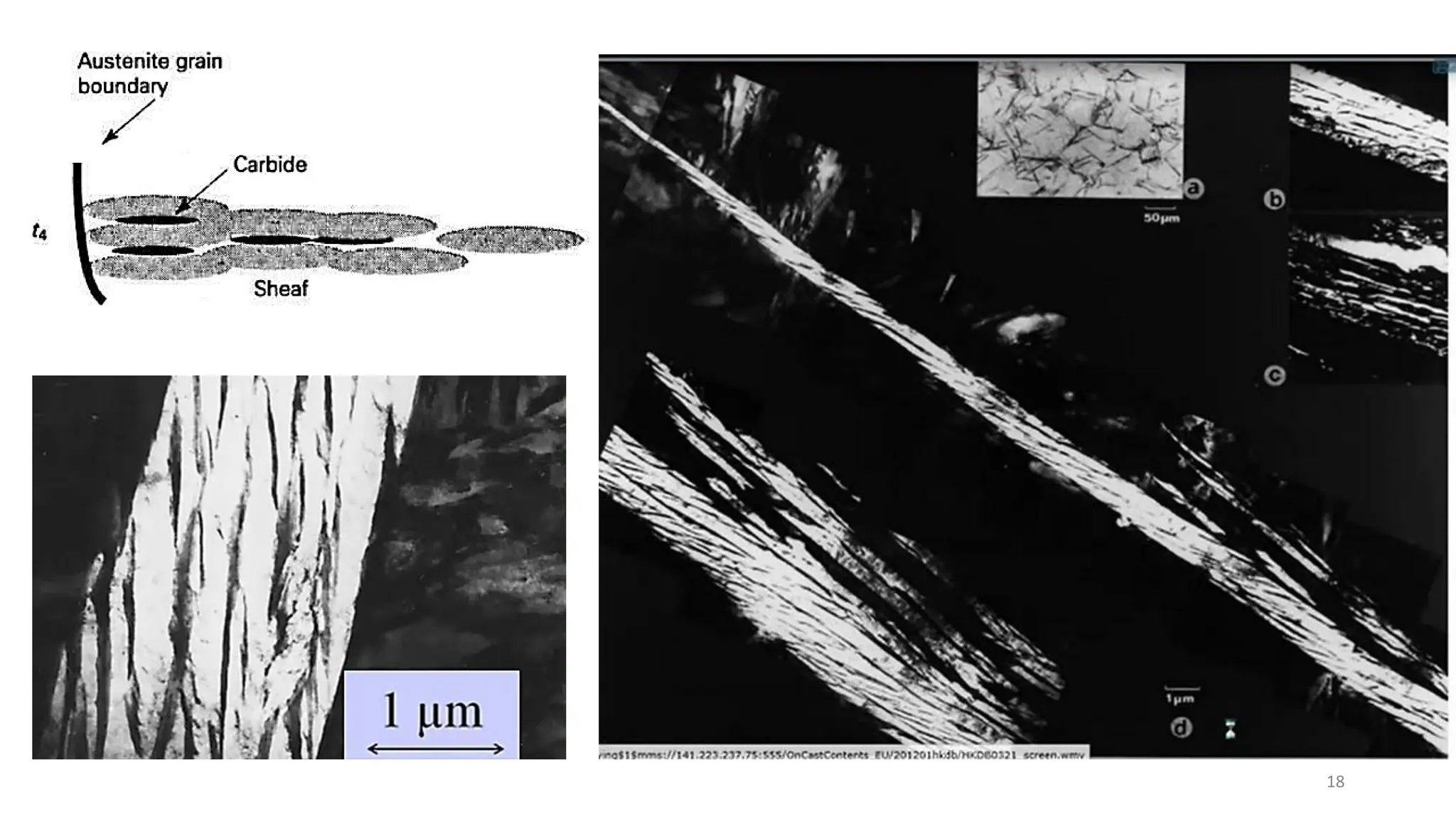
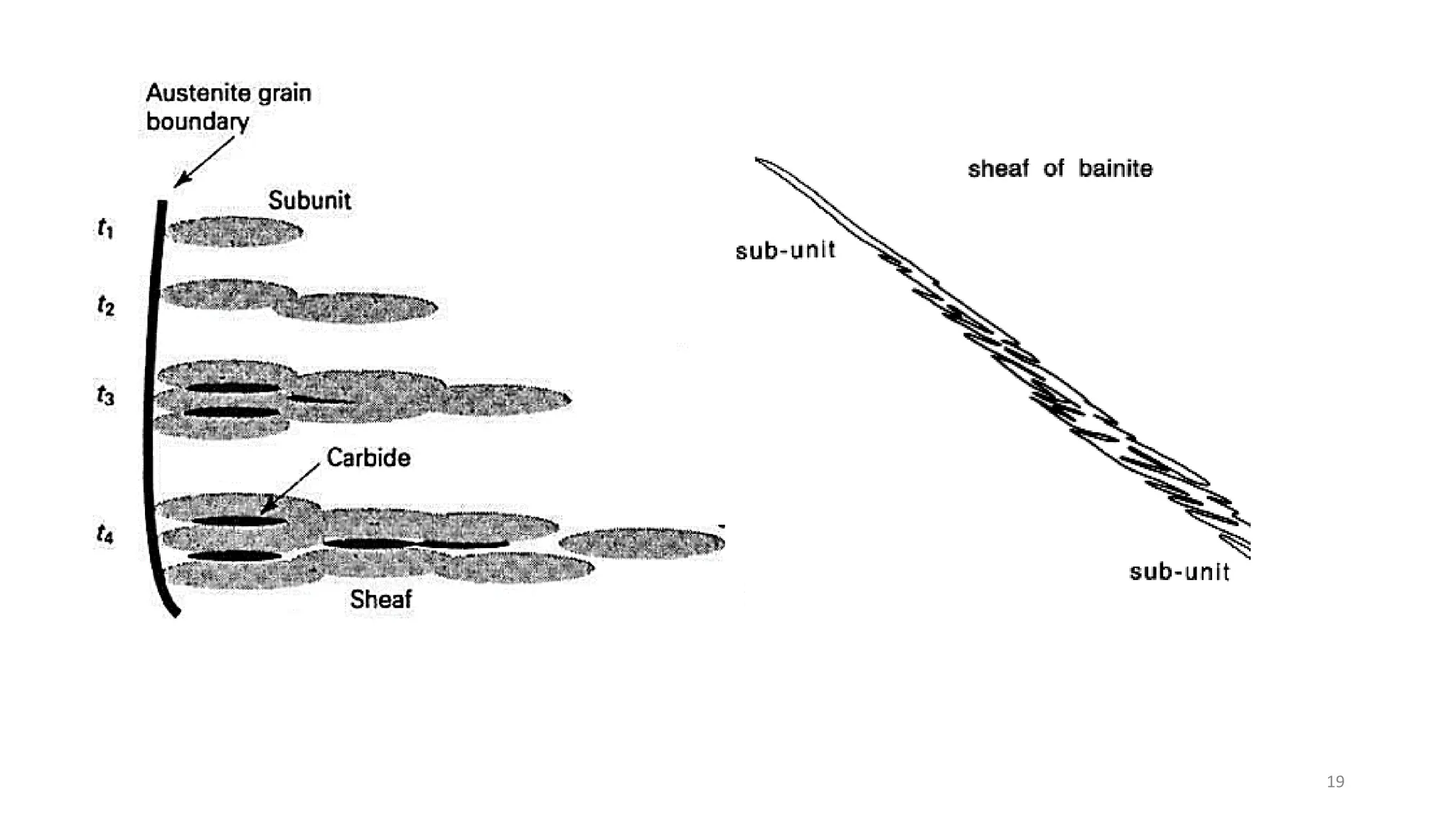
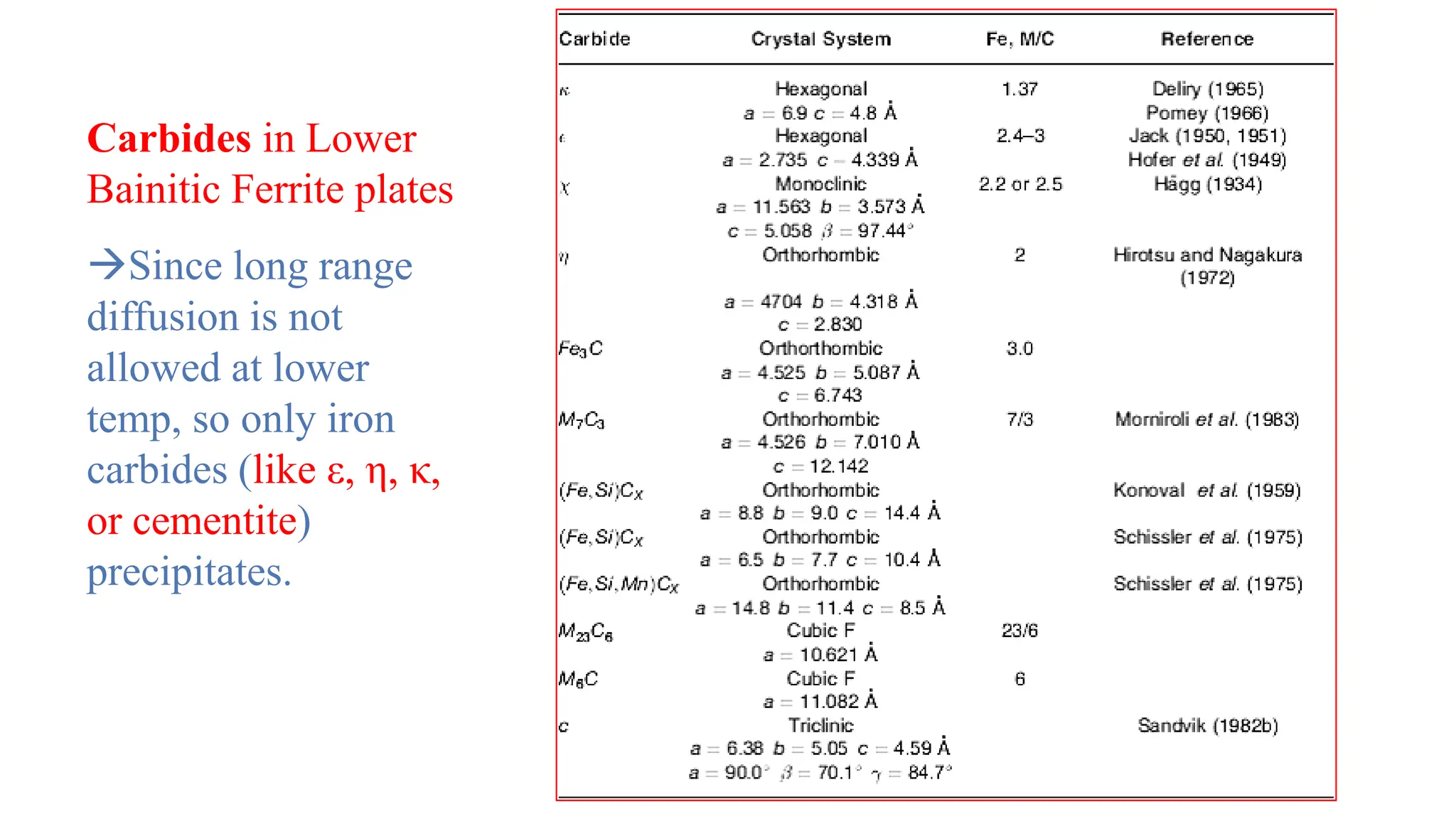
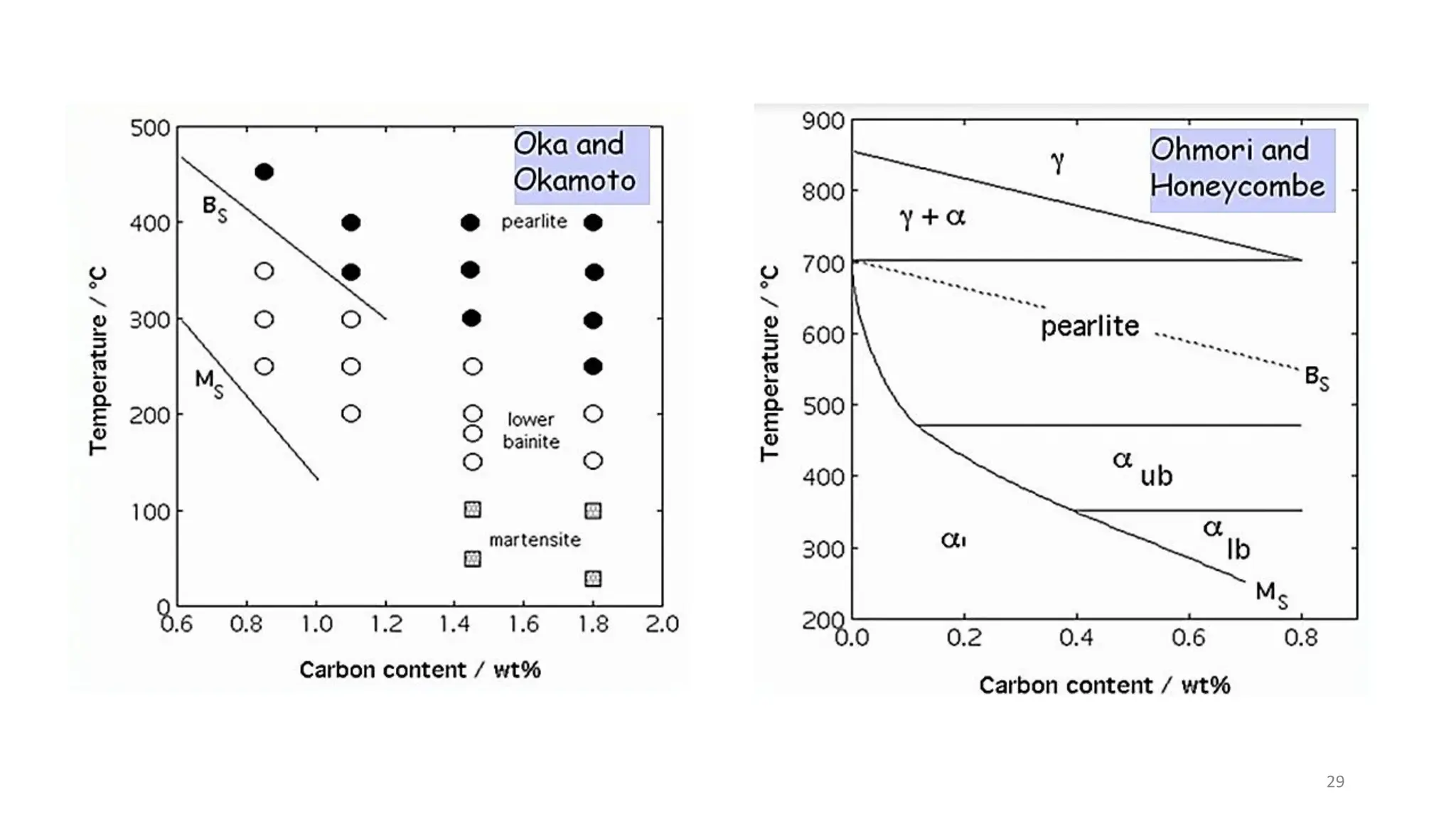
2) Bainite forms by the decomposition of austenite at a temperature above the martensite start temperature but below the temperature at which fine pearlite forms.
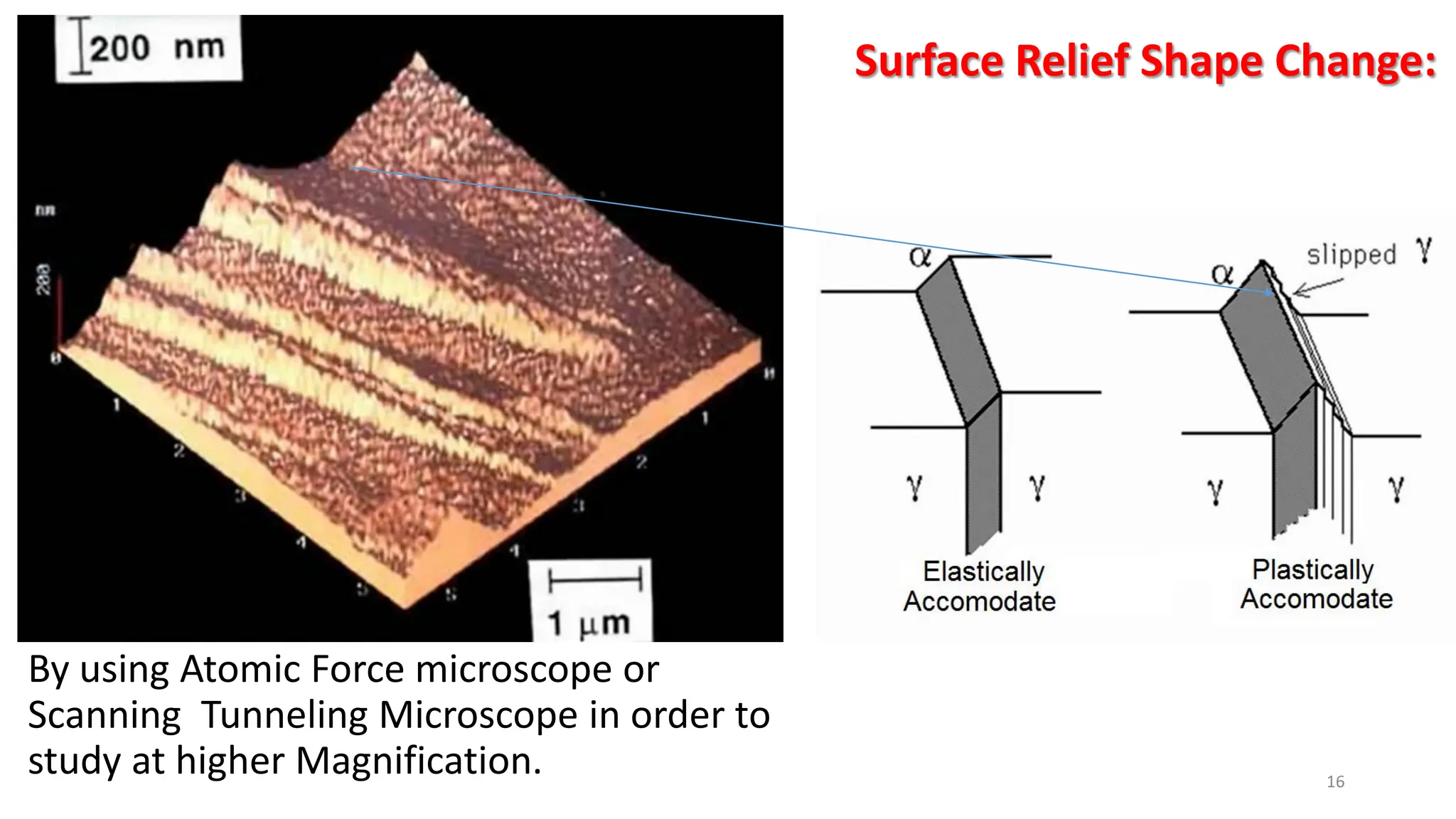
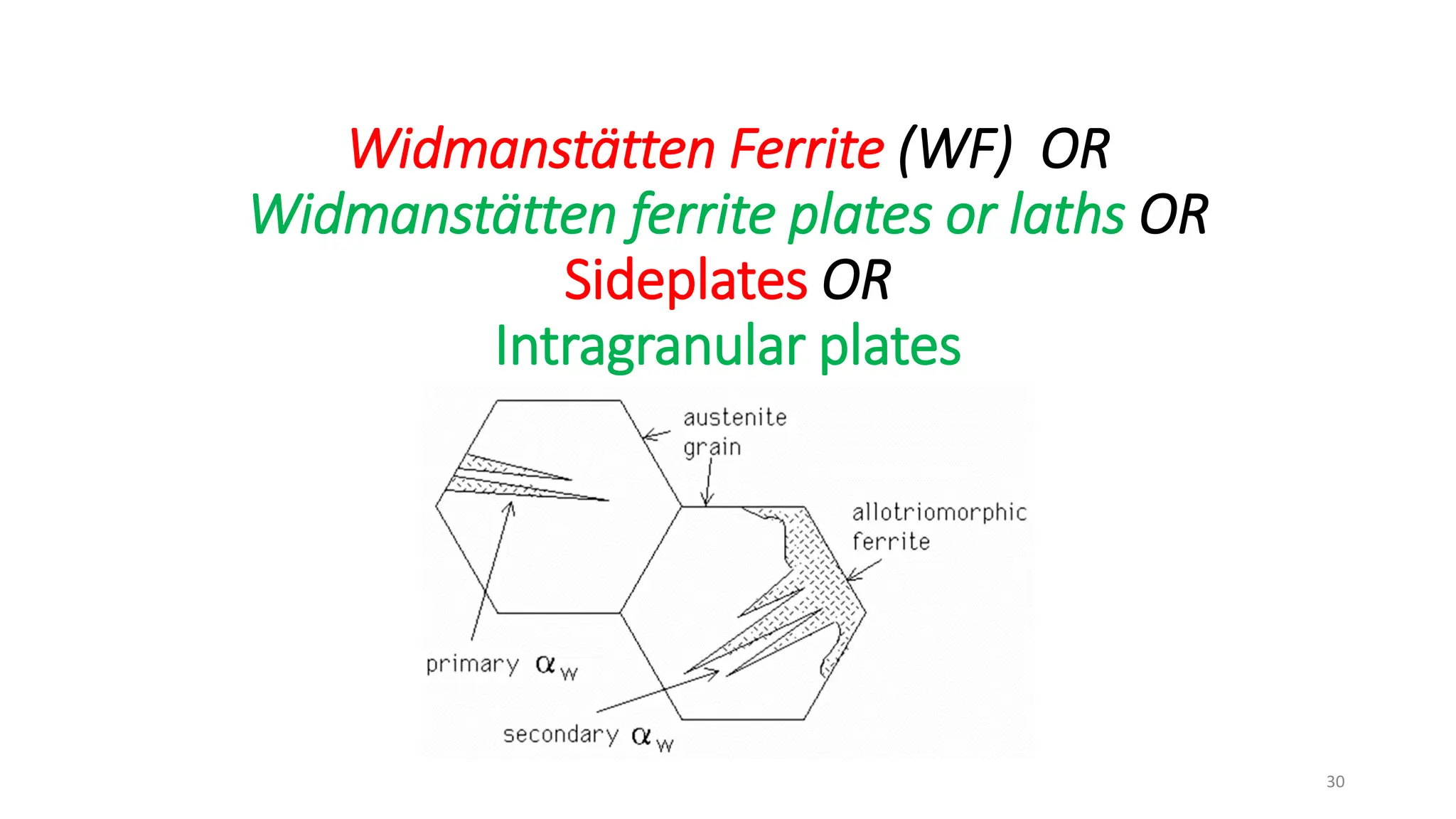
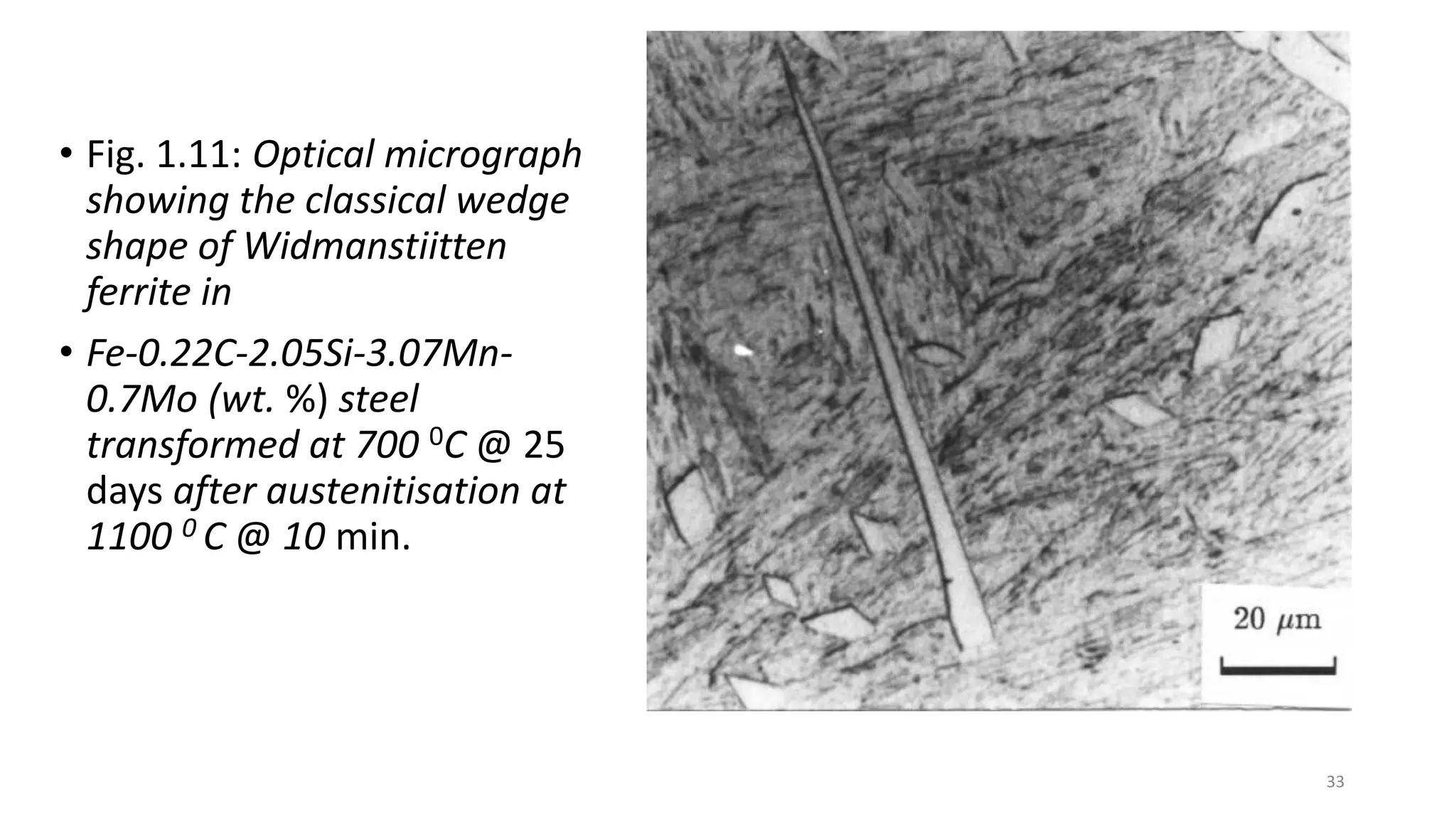
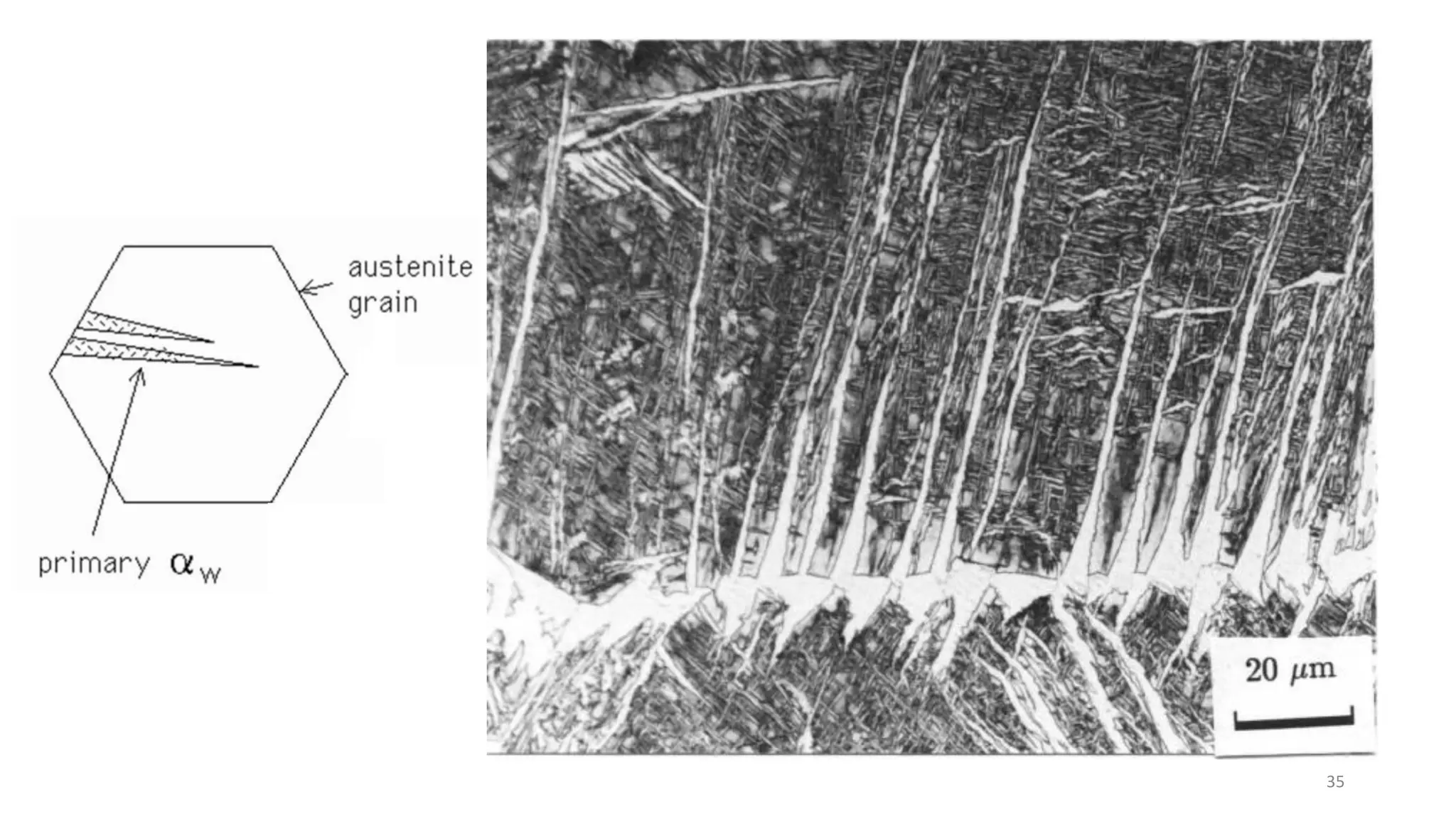
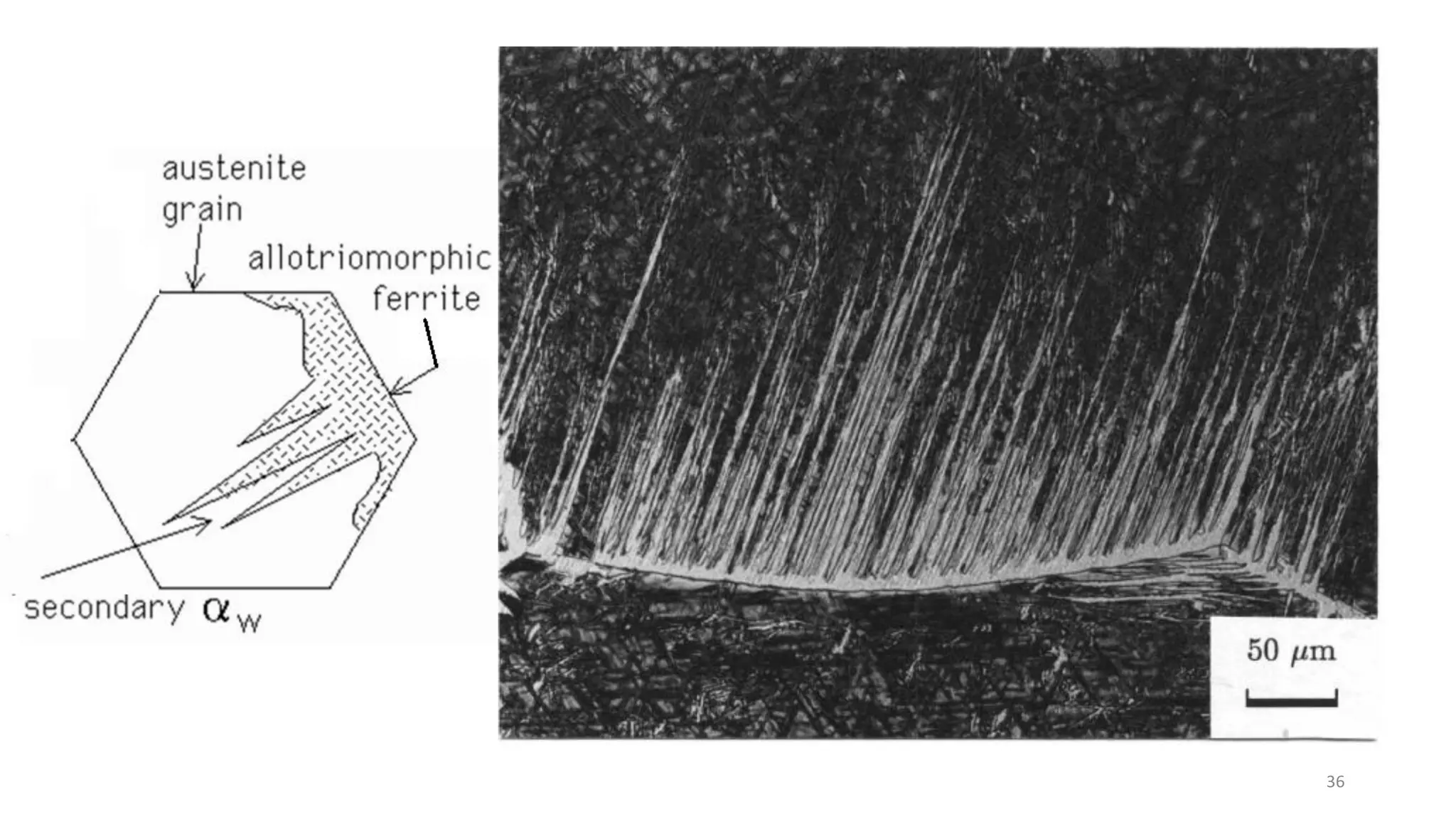
3) Widmanstätten ferrite nucleates from austenite grain boundaries and grows in a displacive mechanism, maintaining an atomic correspondence between the parent and product phases resulting in a triangular shape.
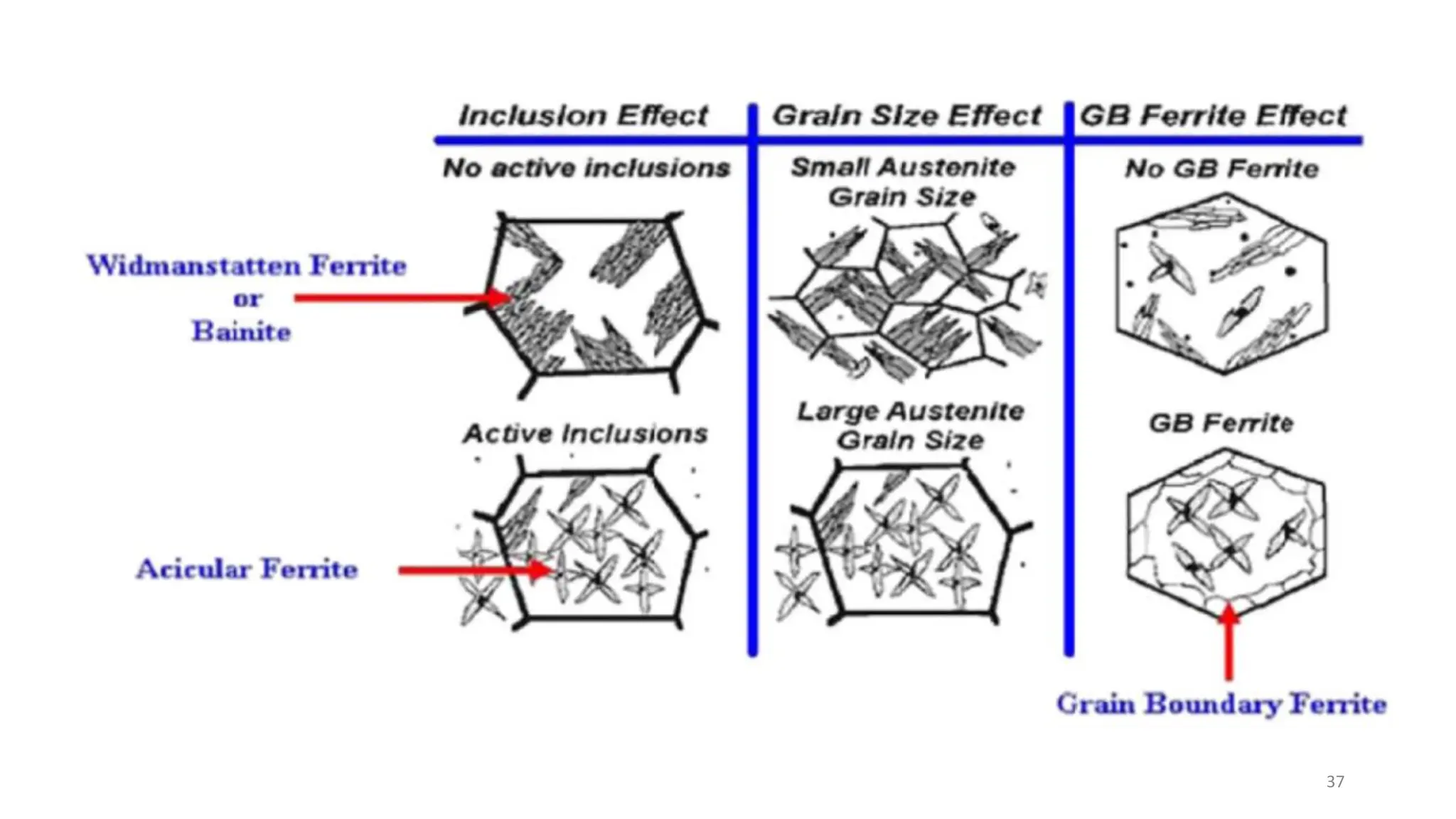
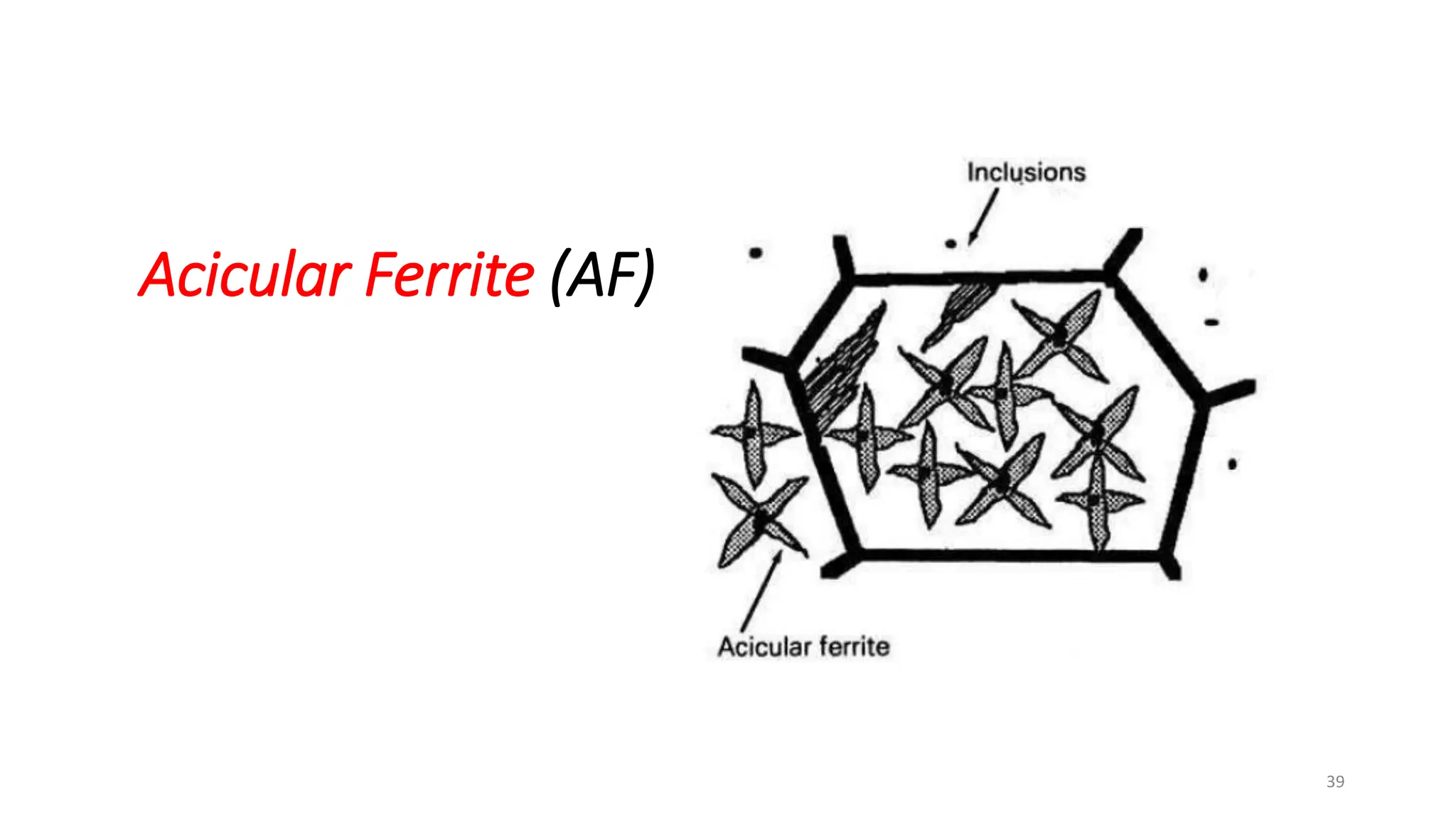
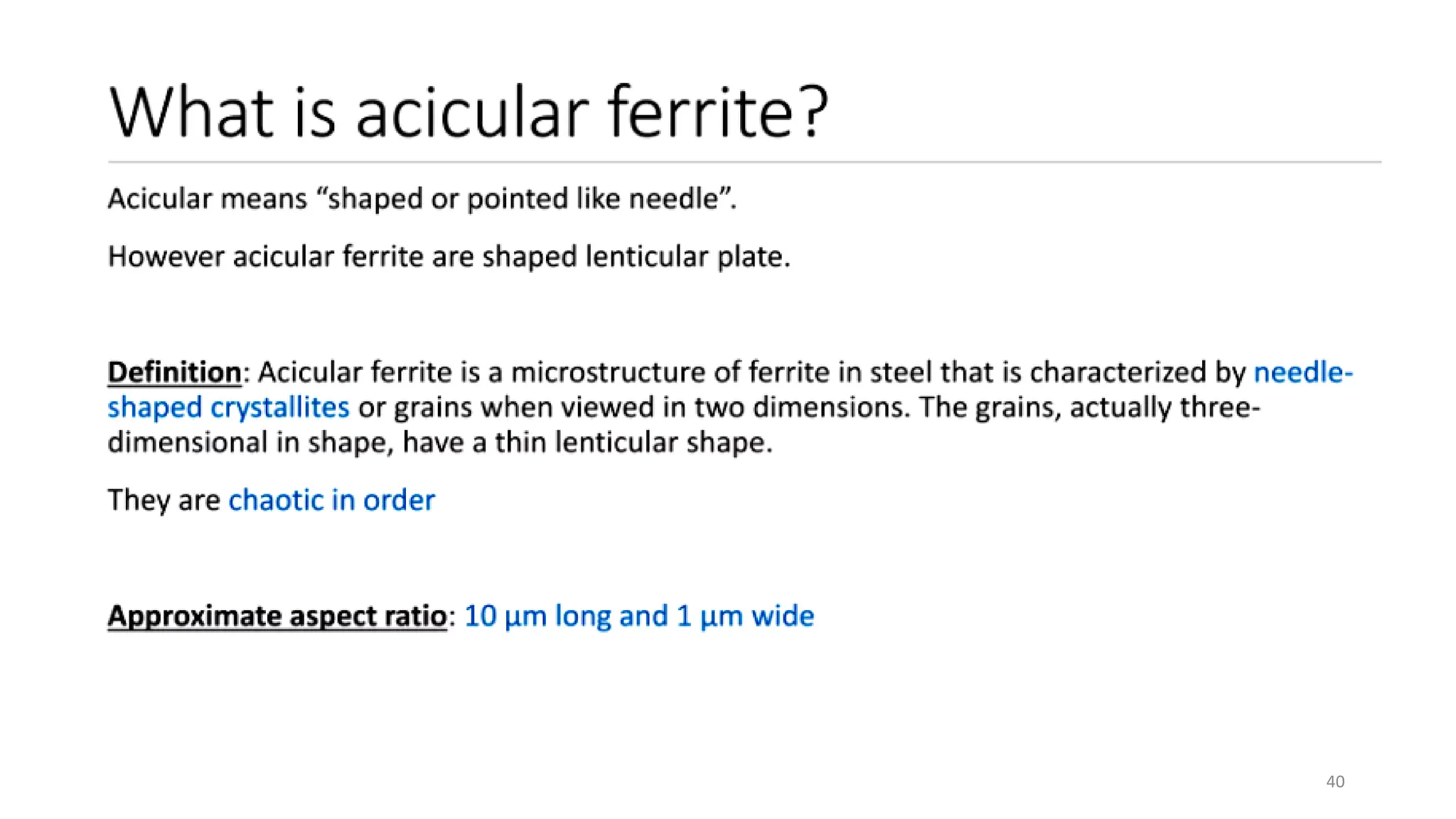
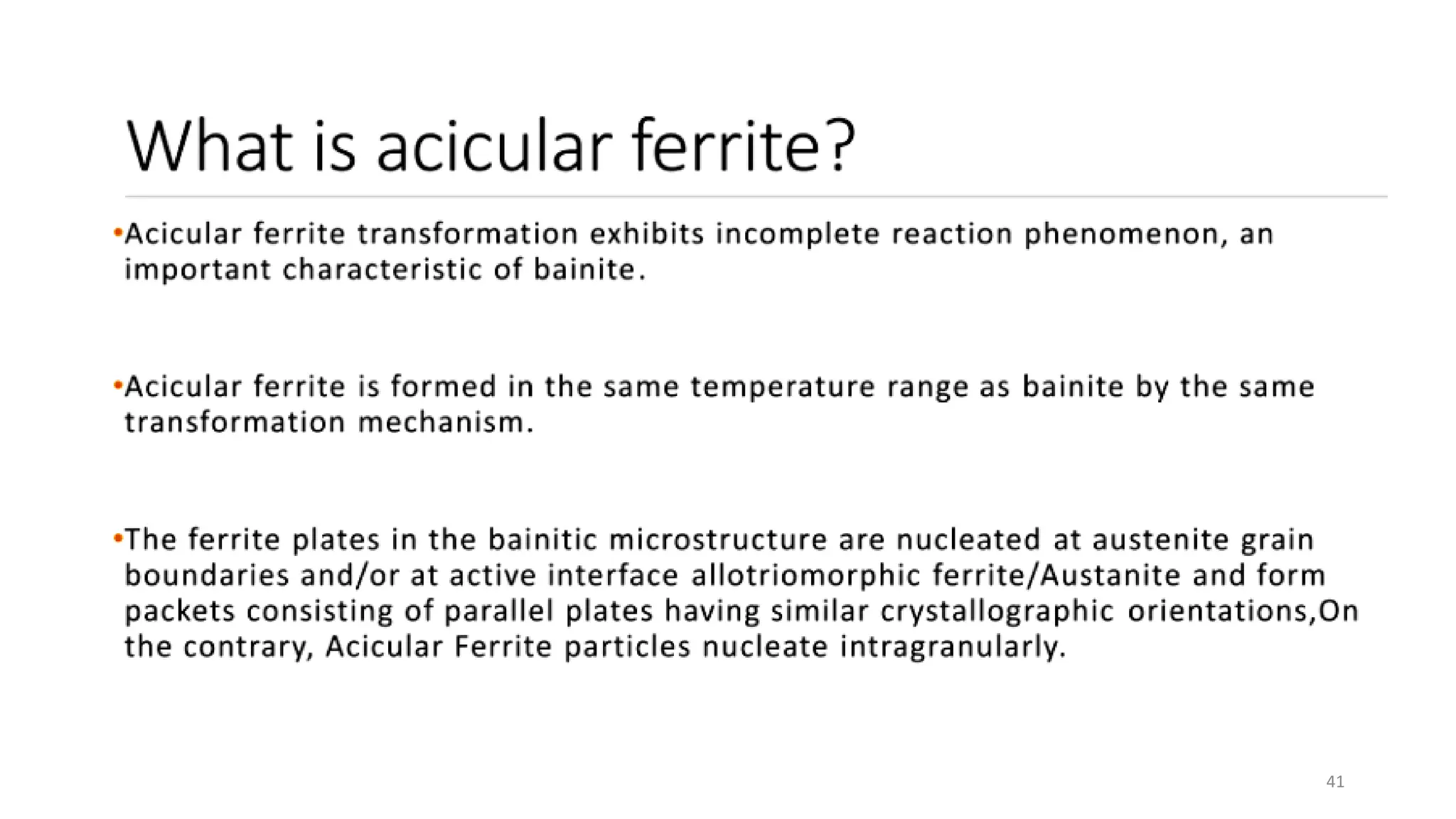
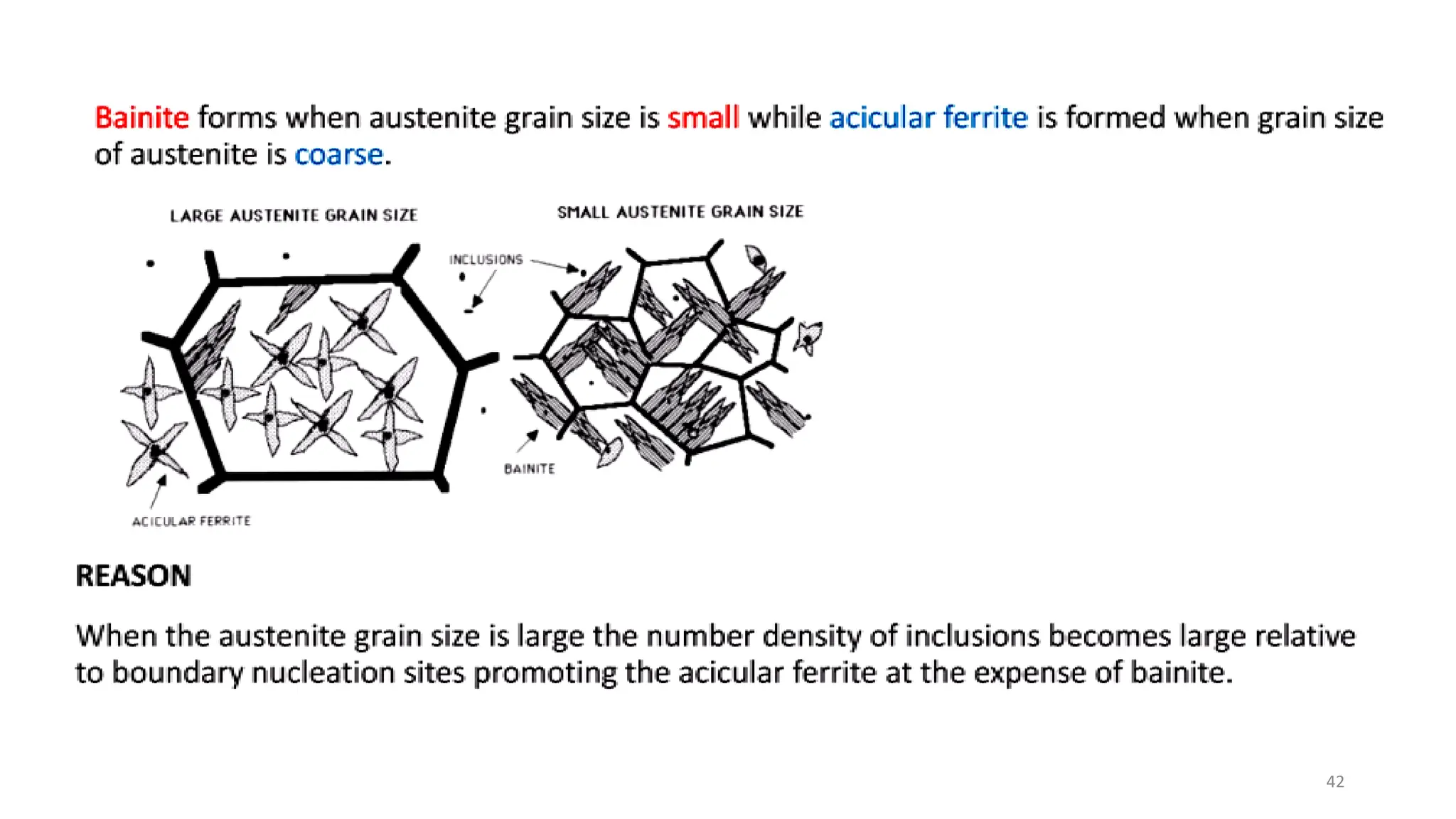
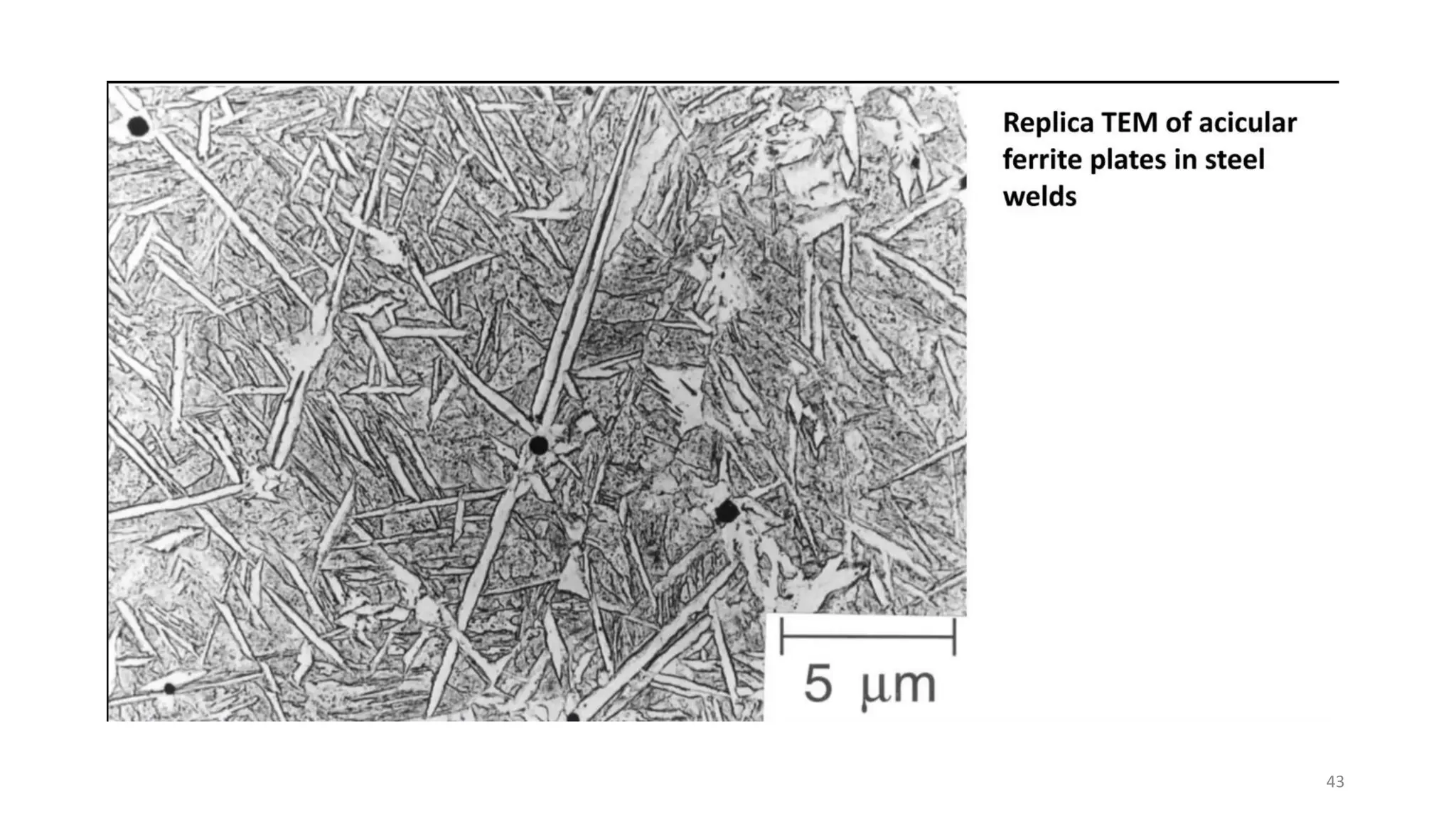
4) Acicular ferrite forms as thin plates within prior austenite grains or as sideplates from grain boundaries