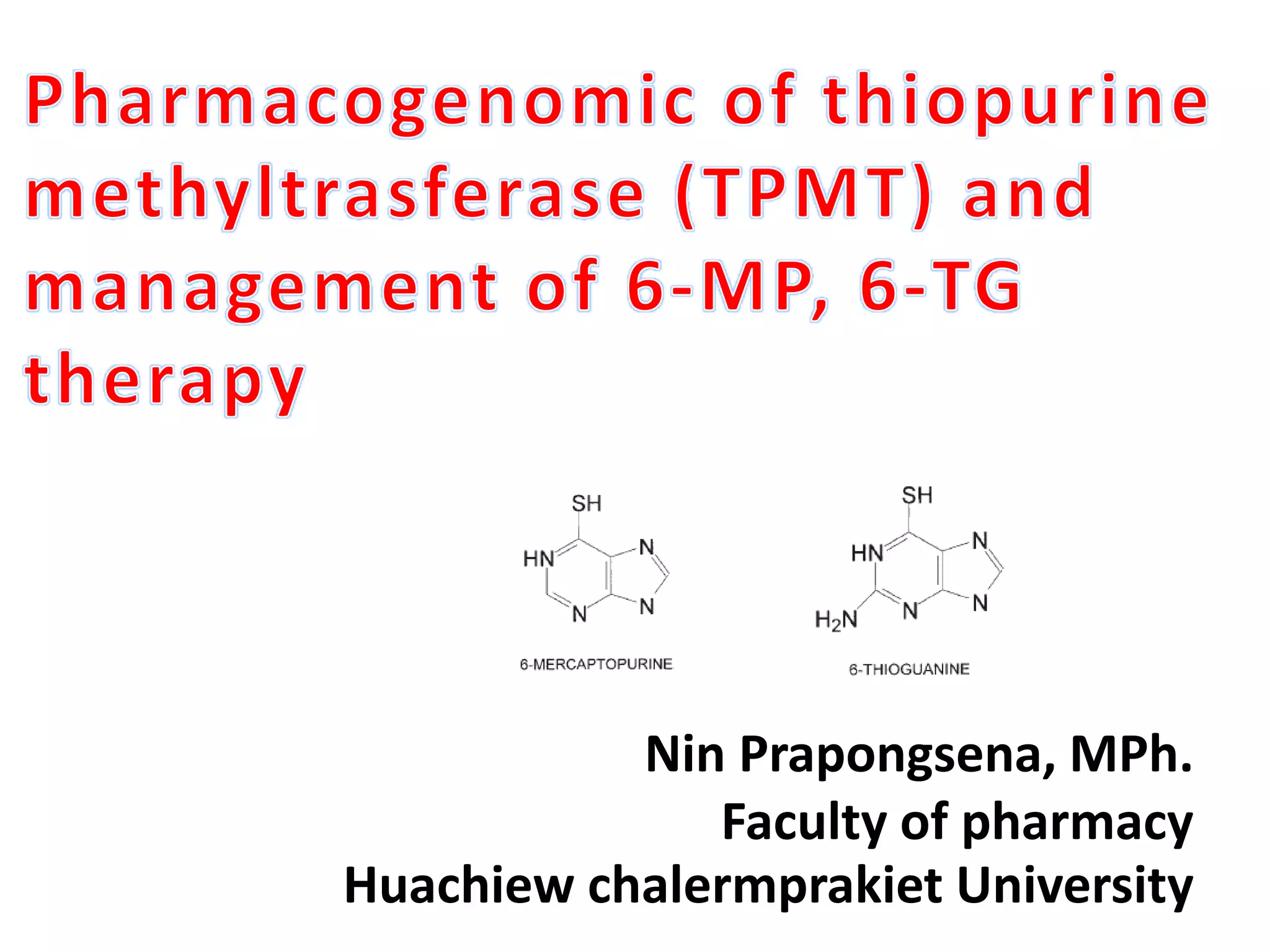
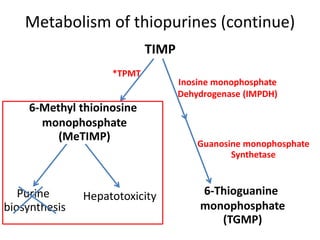
This document outlines a presentation on thiopurine drugs including azathioprine, 6-mercaptopurine, and 6-thioguanine. It discusses their metabolism, mechanisms of action, adverse drug reactions, and importance of testing for thiopurine S-methyltransferase (TPMT) polymorphisms. TPMT activity affects levels of active drug metabolites and risk of toxicity. Genotype correlates with but does not perfectly predict phenotype. The presentation emphasizes dose adjustment based on TPMT levels and close monitoring to improve outcomes with thiopurine treatment.