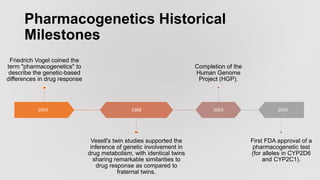
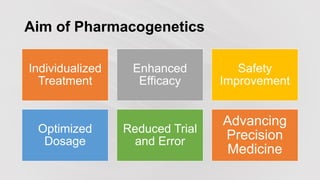
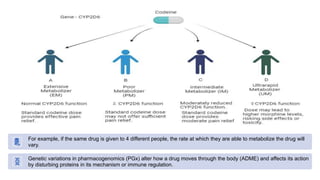
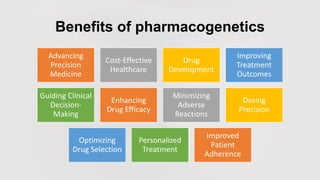
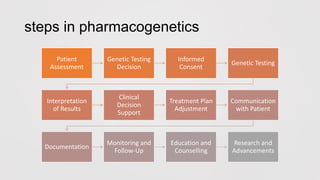
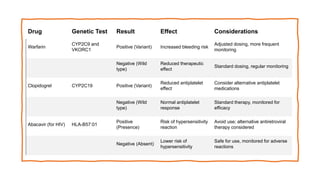
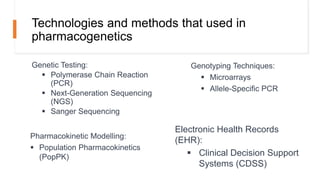
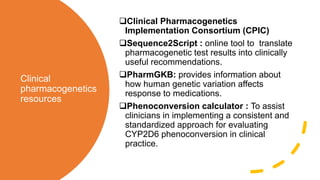
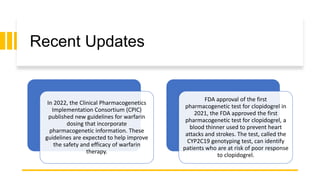
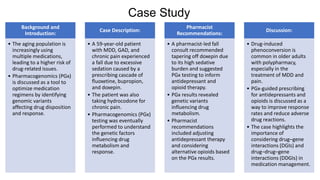
Pharmacogenetics is the study of how an individual's genetic makeup influences their response to drugs. It allows for a personalized medication approach to optimize drug selection and dosing based on genetic factors. Integrating pharmacogenomics into clinical decision making can streamline treatment by minimizing adverse events and improving outcomes through more effective strategies. Proactive pharmacogenetic testing holds promise in preventing medication issues by offering a personalized, preventative approach to care. While challenges remain, advancements in technology and research are advancing pharmacogenetics' role in precision medicine.