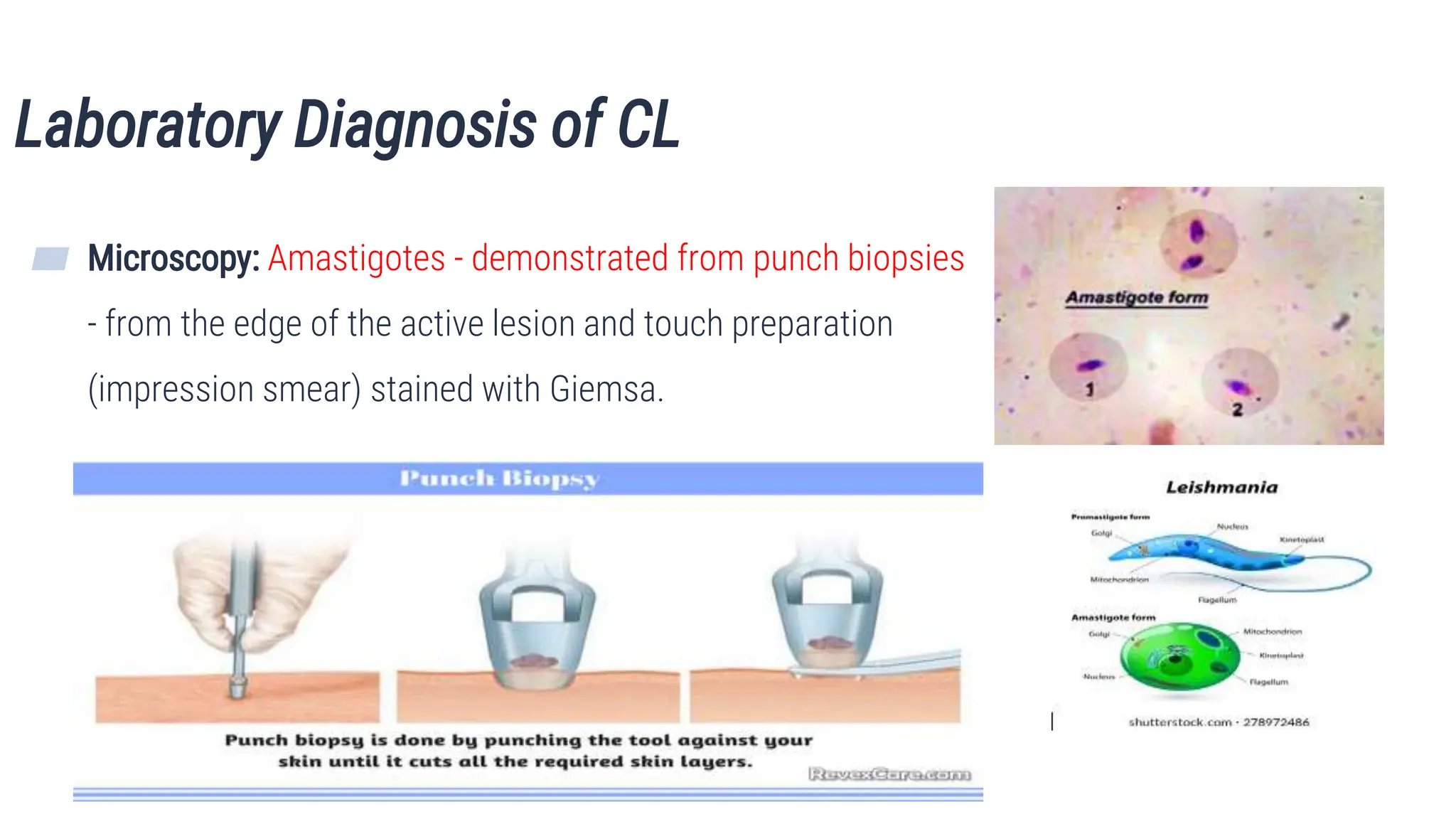
The document discusses various parasitic infections affecting the skin, soft tissue, and musculoskeletal system, detailing their lifecycle, clinical features, diagnosis, treatment, and prevention. Key infections covered include cutaneous leishmaniasis, filariasis, dracunculiasis, and others, each with distinct causative agents and manifestations. The document aims to educate students about these infections to enhance their understanding and management in medical practice.