1) The document provides an overview of paracetamol (acetaminophen), including its history, pharmacokinetics, mechanism of action, toxicity, diagnosis, treatment, and prognosis.
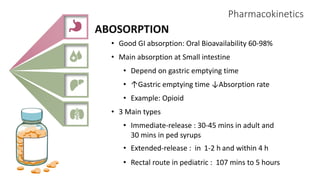
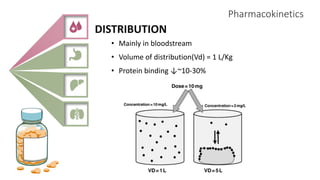
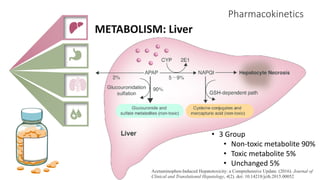
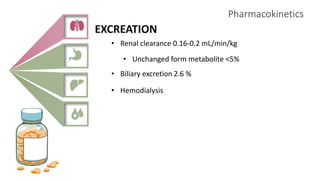
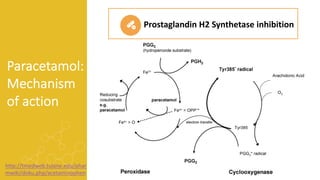
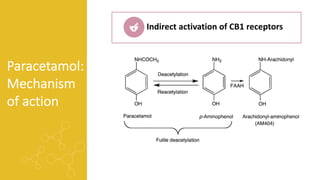
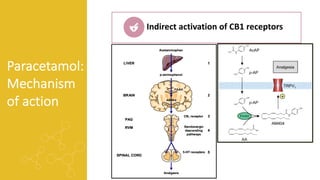
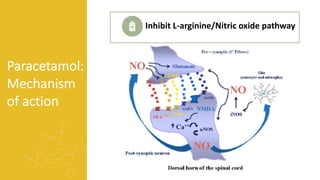
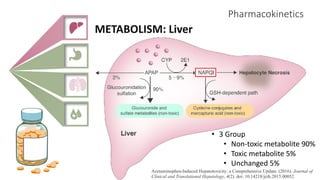
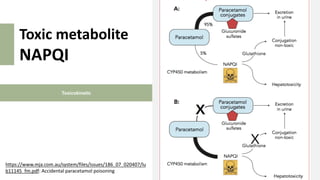
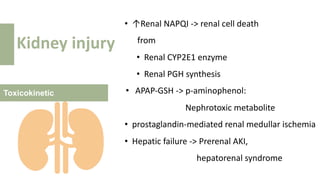
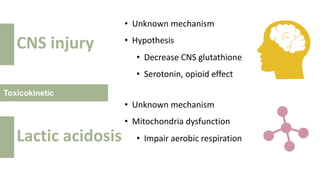
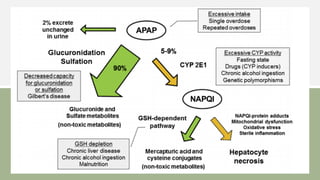
2) It describes paracetamol's absorption in the small intestine, metabolism primarily in the liver, and excretion mainly through the kidneys. Toxicity can occur when a toxic metabolite, NAPQI, is formed and not detoxified.
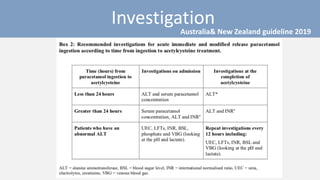
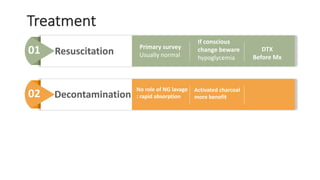
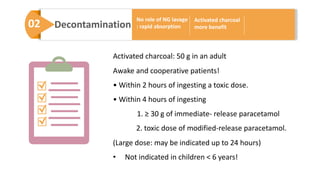
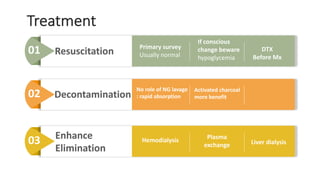
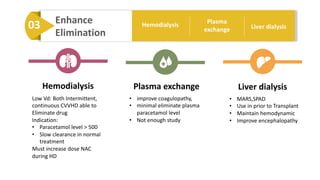
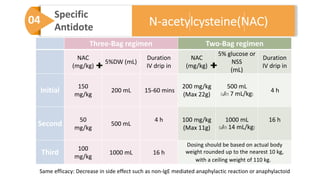
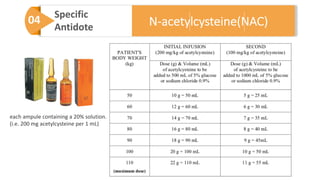
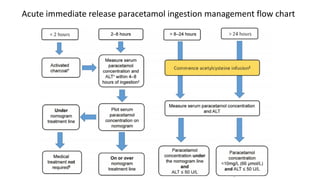
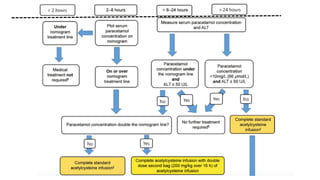
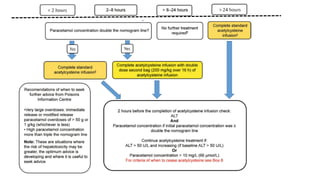
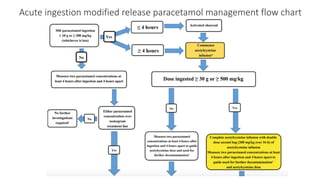
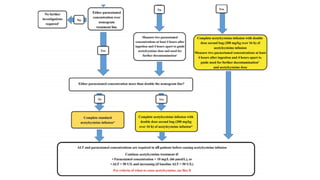
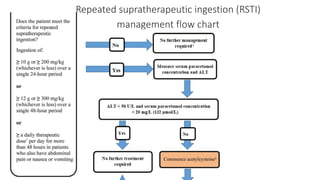
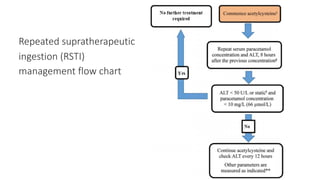
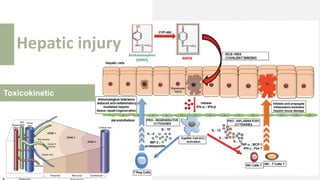
3) Treatment for paracetamol overdose involves gastric decontamination, administration of the antidote N-acetylcysteine to replenish glutathione levels and prevent liver damage, and potentially hemodialysis or liver transplant for




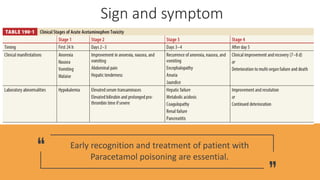
![Tintinali’s Goldfrank’s Australia CPG
Single Dose
Adult>6 yrs >10 g, > 200 mg/kg >7.5 gm >10g, >200mg/kg
Child<6 yrs >200 mg/kg >150 mg/kg >200 mg/kg
RSTI
Adult>6 yrs >10g,>200mg/kg
in 24 h
>10g,>200mg/kg
in 24h
>10g,>200mg/kg
in 24 h
>6g,
>150mg/kg
in 24 h x 2days
>6g,
>150mg/kg
in 24 h x 2days
>12g,
>300 mg/kg
in 48 h
>60mg/kg[>4g] in48 h
+ clinical
Child<6 yrs
>200 mg/kg in 8h
>150 mg/kg
in 24 x2 days
>100 mg/kg/d in 72h Massive >= 30 g](https://image.slidesharecdn.com/para-200511175525/85/Paracetamol-overdose-24-320.jpg)
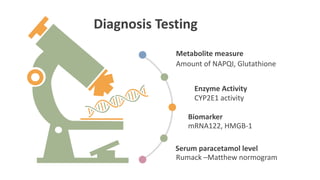
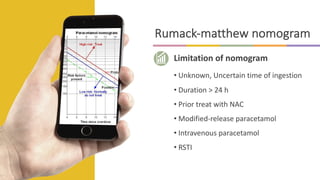
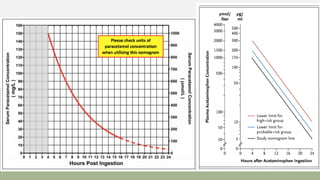
![Acetaminophen-Aminotransferase
multiplication product [APxAT]
< 1500 mg.IU/L
1500-10000 mg.IU/L
No need treatment.
Low risk
- Use AST or ALT which is higher
- If no paracetamol level use 5 instead
Possible
> 10,000 mg.IU/L Probable
Paracetamol level x AST/ALT](https://image.slidesharecdn.com/para-200511175525/85/Paracetamol-overdose-31-320.jpg)