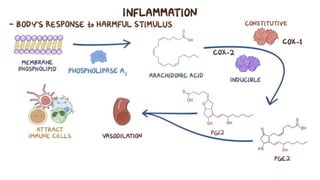
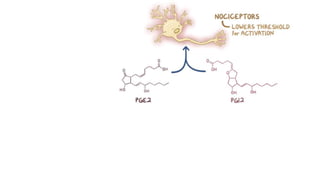
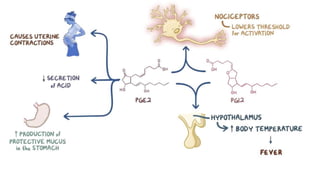
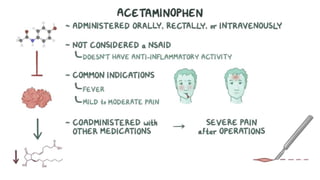
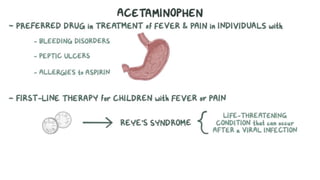
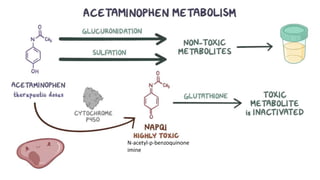
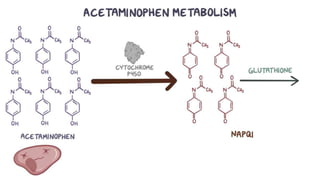
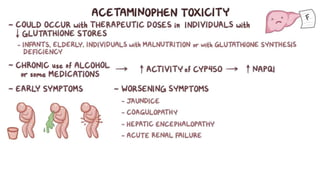
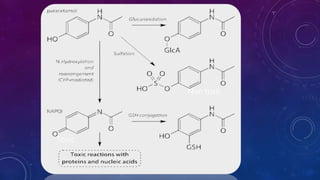
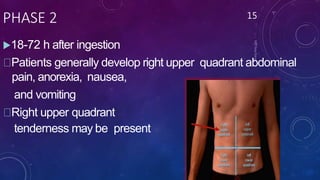
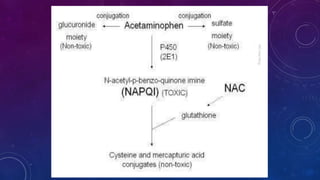
Paracetamol was developed in the late 1880s as a less toxic derivative of acetanilide. It is metabolized in the liver to a toxic intermediate called NAPQI, which is normally detoxified by glutathione. An overdose can deplete glutathione stores, allowing NAPQI to damage liver cells. Paracetamol poisoning has few early symptoms but can cause liver failure and death. Treatment involves N-acetylcysteine to replenish glutathione within 8-24 hours of ingestion. Prognosis depends on factors like time to treatment, dose ingested, and underlying liver health.