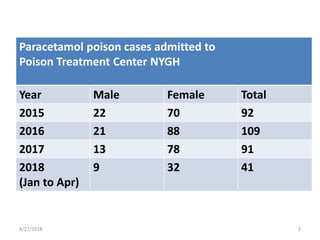
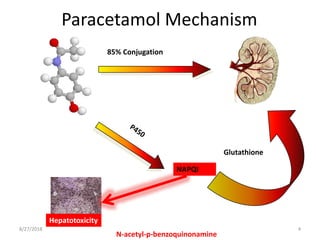
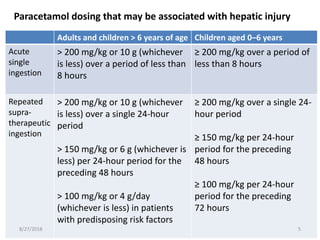
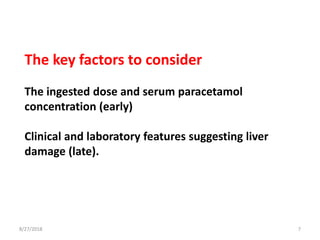
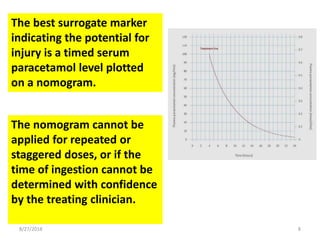
This document summarizes information about paracetamol poisoning, including statistics on cases from 2015-2018 at a poison treatment center. It describes the absorption, distribution, and metabolism of paracetamol. Threshold doses for potential hepatic injury are provided for both single and repeated ingestions in adults, children, and high-risk groups. The clinical features and phases of paracetamol poisoning are outlined. Recommended investigations and treatment with N-acetylcysteine via three-stage infusion are summarized, along with potential reactions and alternative oral treatment. Timely treatment with NAC is emphasized as crucial to prevent liver toxicity.