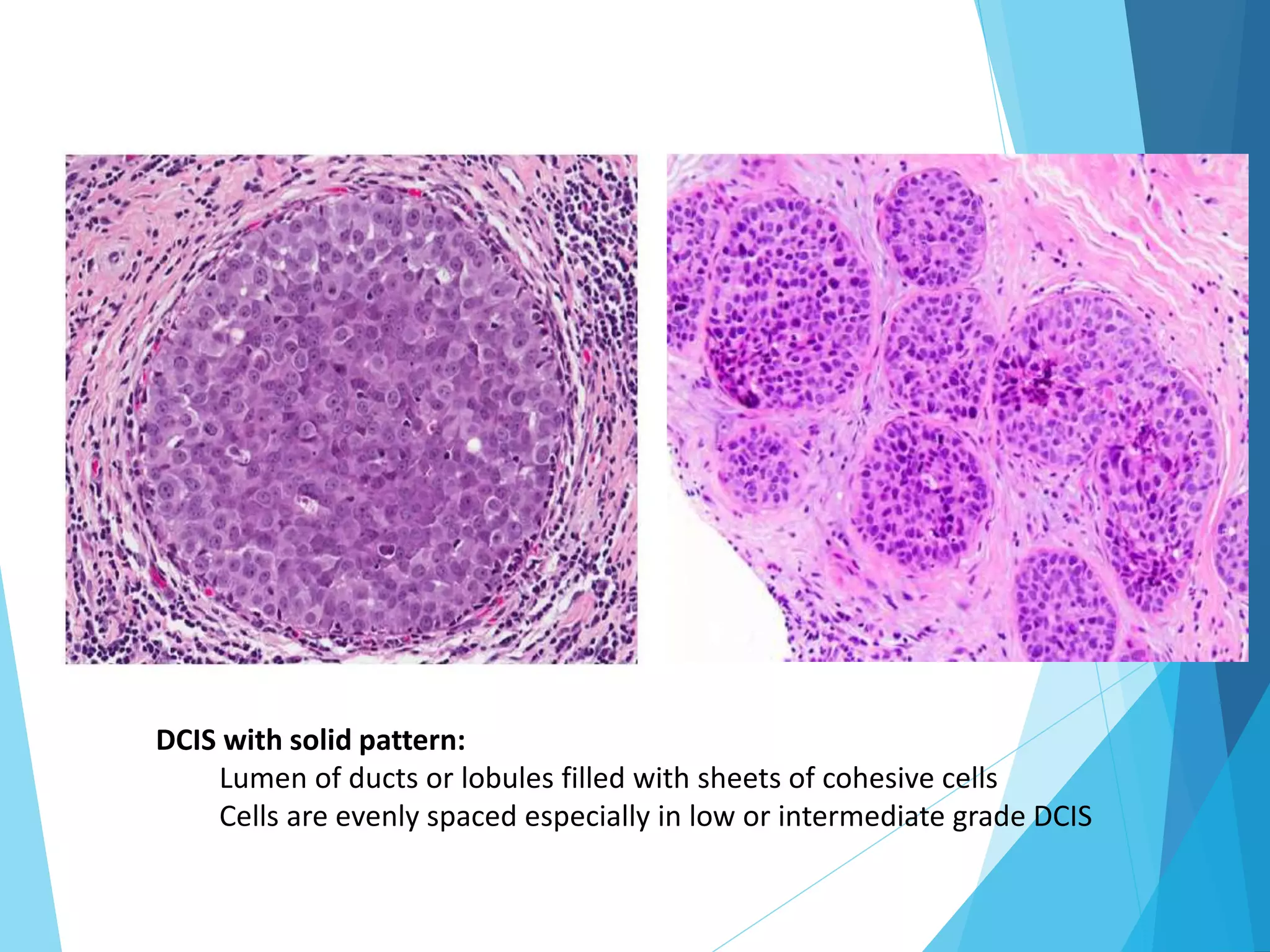
The document discusses the histopathology of breast cancer, detailing the structure of the female breast, types of benign and malignant lesions, and classifications of breast carcinoma. It covers the progression from non-proliferative breast changes to various forms of carcinoma, emphasizing the importance of molecular classification for prognosis and treatment. Additionally, it reviews diagnostic approaches and the 'triple test' method for accurately classifying breast masses.