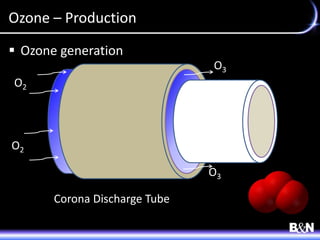
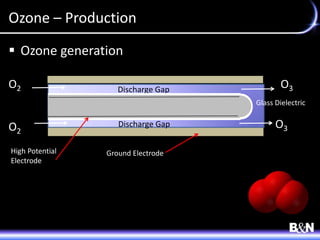
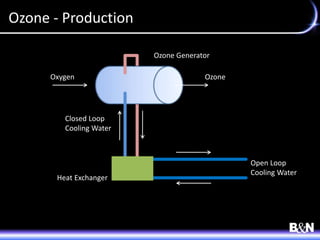
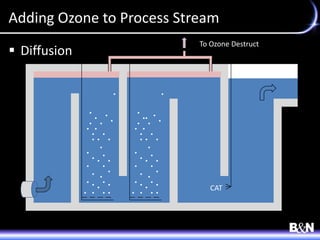
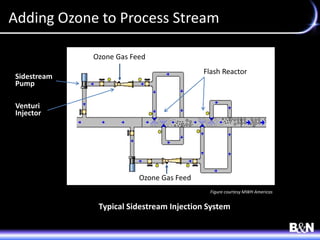
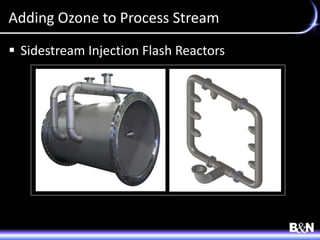
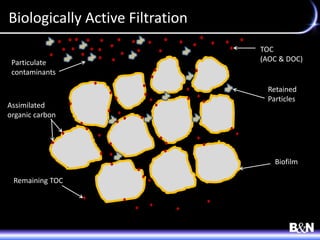
This document discusses ozone and biologically active filtration for controlling disinfection byproducts in drinking water treatment. It provides an overview of ozone production, application, safety considerations, and operational design. Biologically active filtration is described as using the same rapid sand filtration concepts while also removing assimilable organic carbon through the establishment of biofilm. The document outlines pilot demonstrations and applications of these technologies in Ohio drinking water facilities.