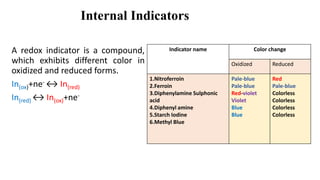
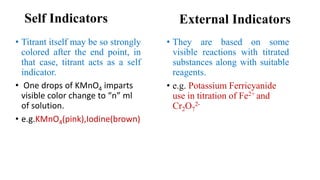
Redox indicators are colorimetric reagents that undergo a distinct color change at specific electrode potentials, categorized into internal, self, and external indicators. Internal indicators exhibit different colors in oxidized and reduced forms, self indicators are strongly colored titrants, and external indicators react with titrated substances. Their applications include pharmaceutical analysis, inorganic and organic compounds determination, and assessing water quality.