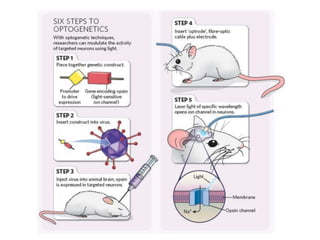
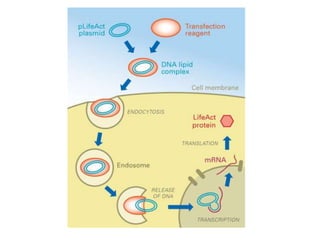
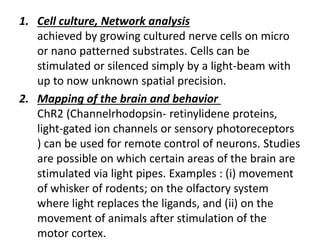
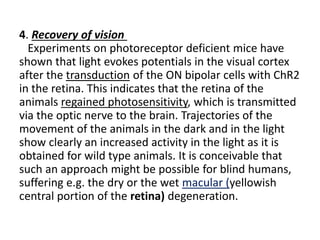
Optogenetics is a technique that uses light to control neurons that have been genetically modified to express light-sensitive ion channels. It allows scientists to precisely stimulate or silence neural activity by exposing specific neurons to light. The first demonstration of optogenetics in mammalian neurons used channelrhodopsin, a light-activated ion channel from algae, to activate neurons with light. Optogenetics holds promise for advancing understanding of brain function and developing new treatments for neurological disorders like Parkinson's disease, epilepsy, and blindness through targeted neuromodulation with light. Challenges include improving light-sensitive tools and light sources to target deeper brain regions.