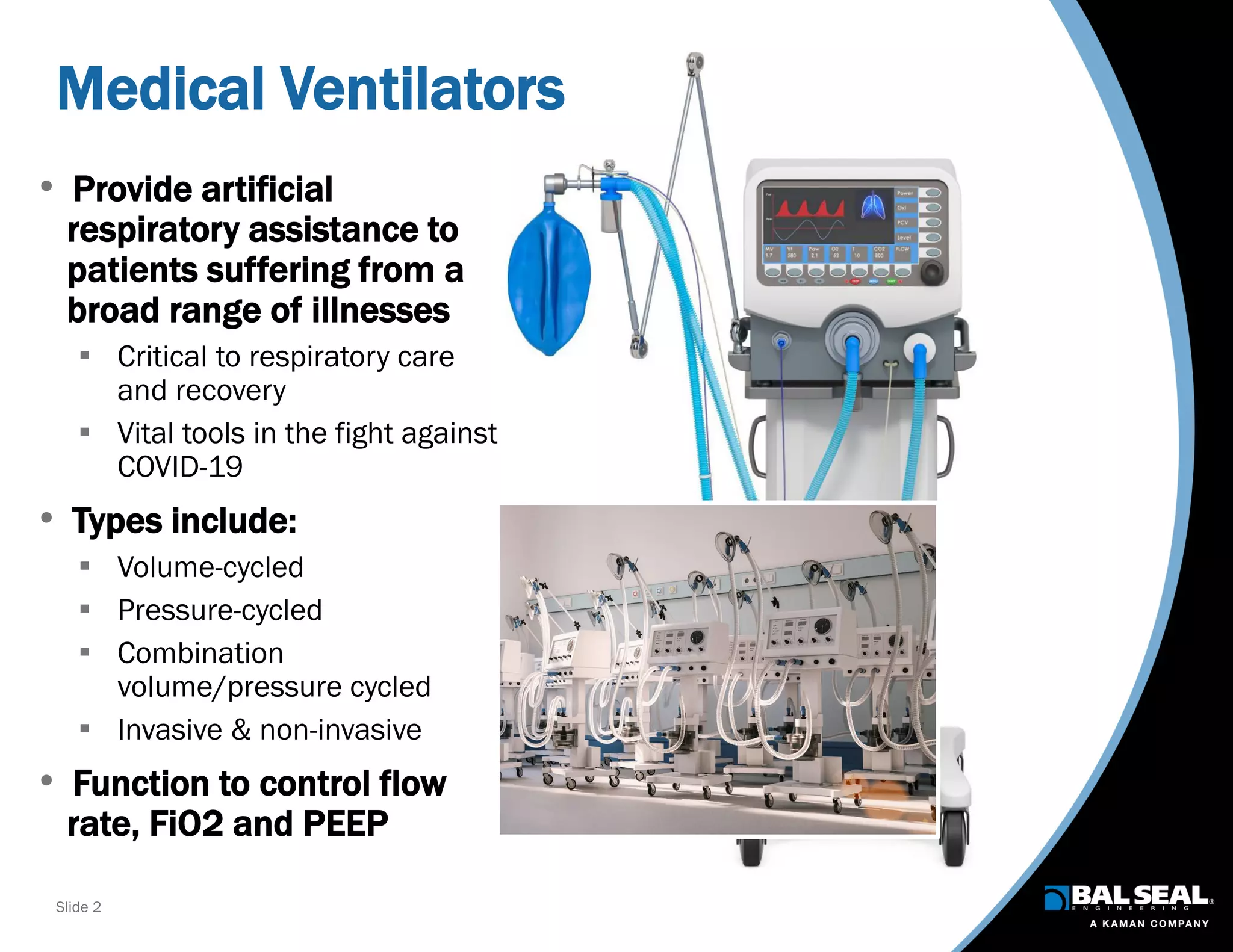
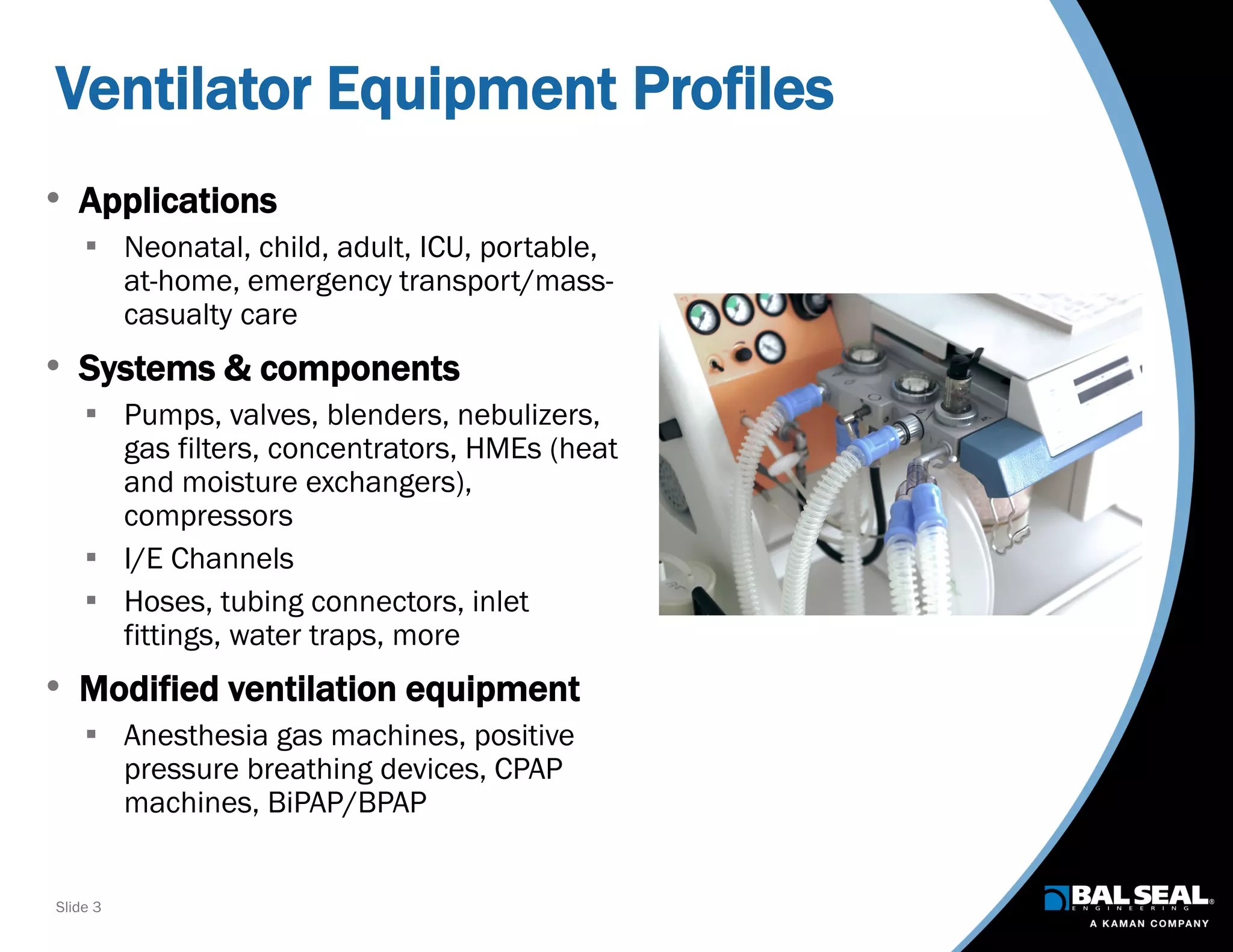
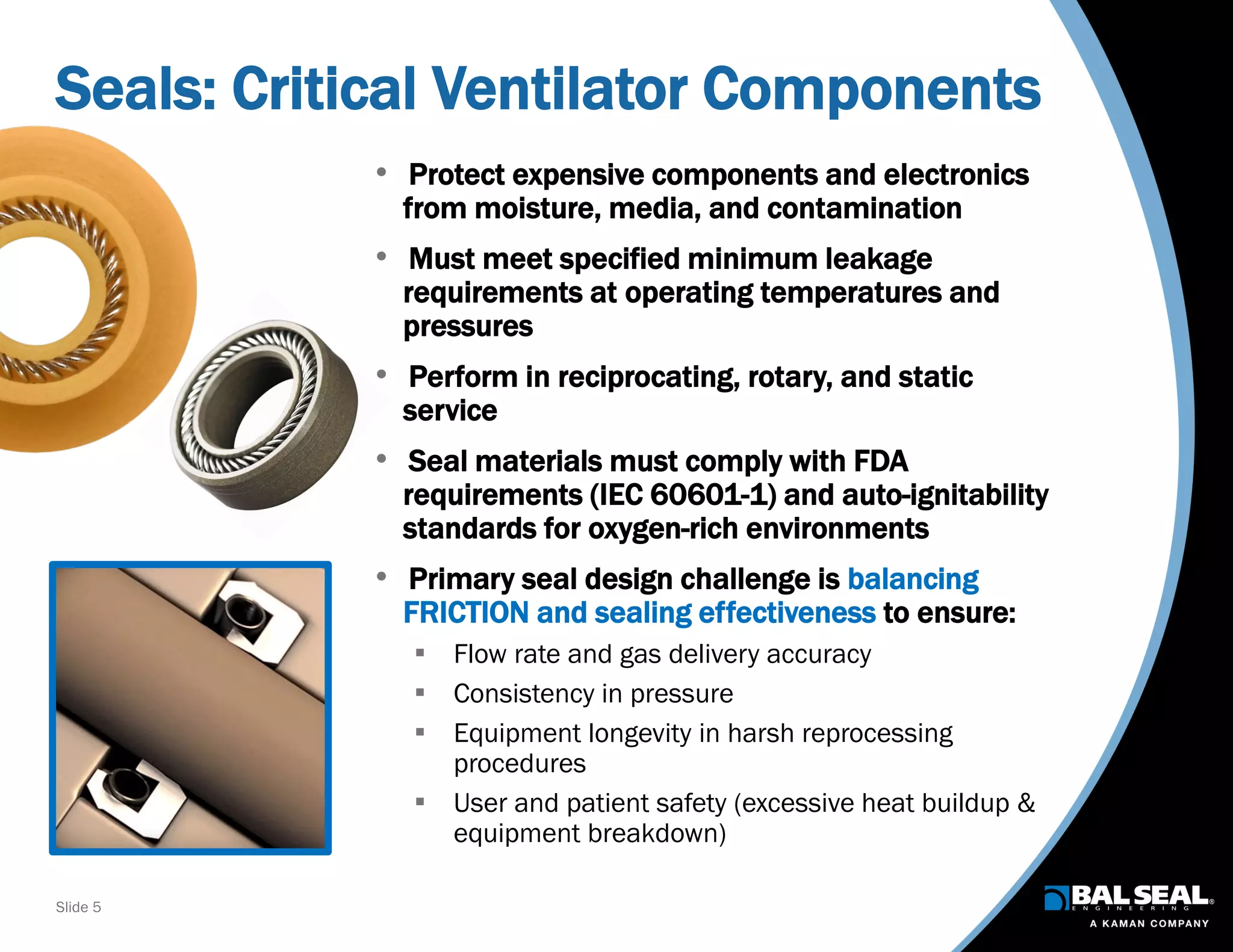
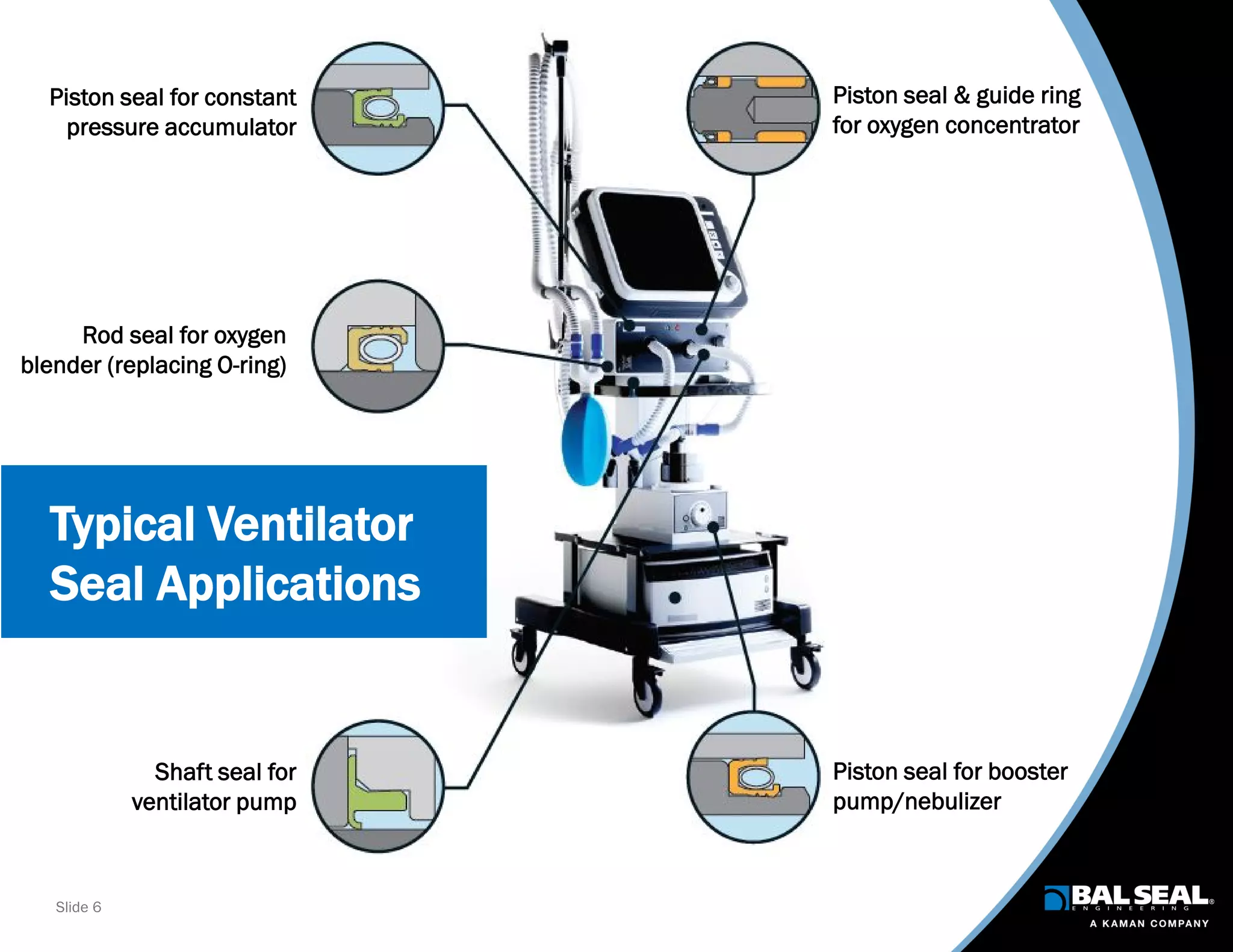
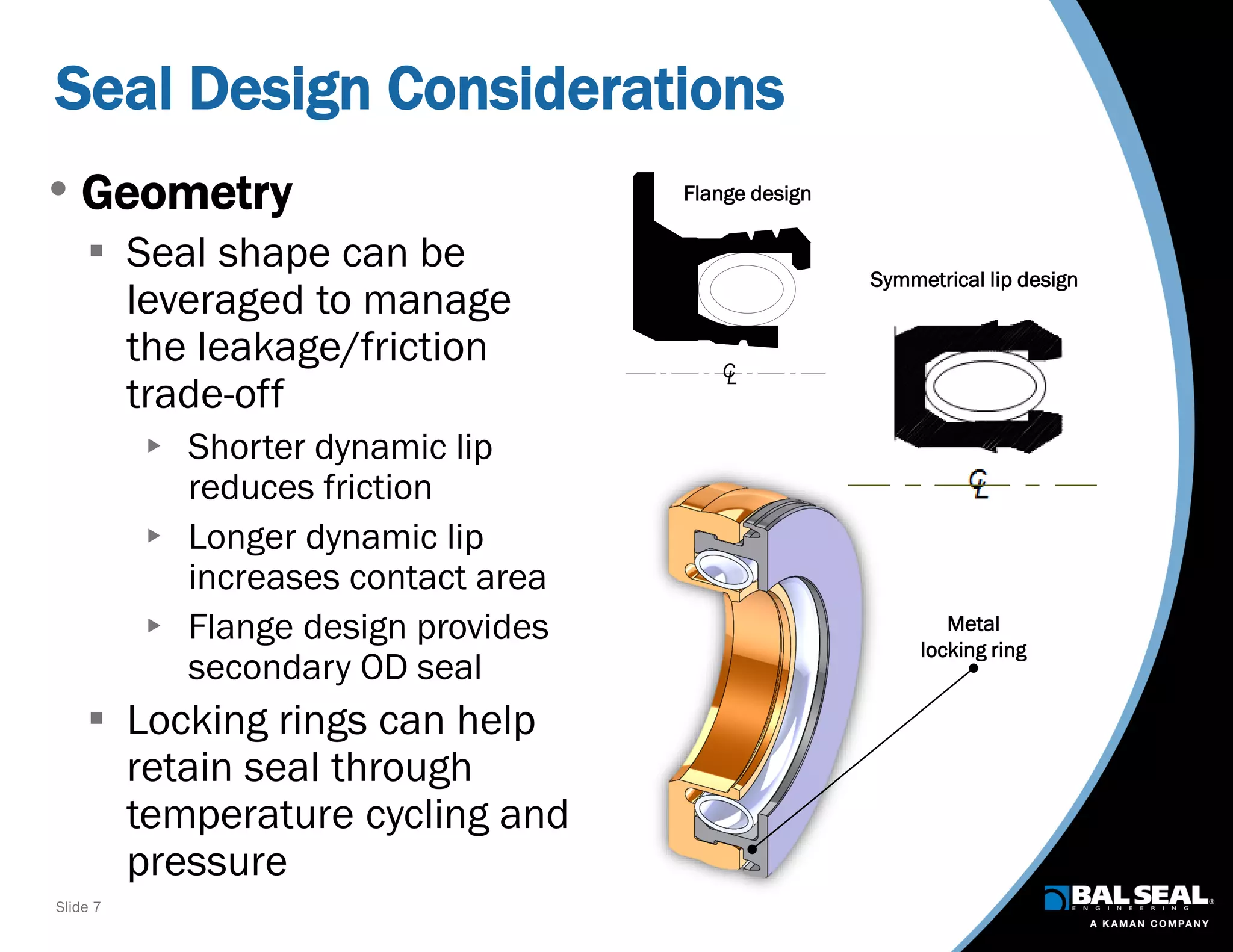
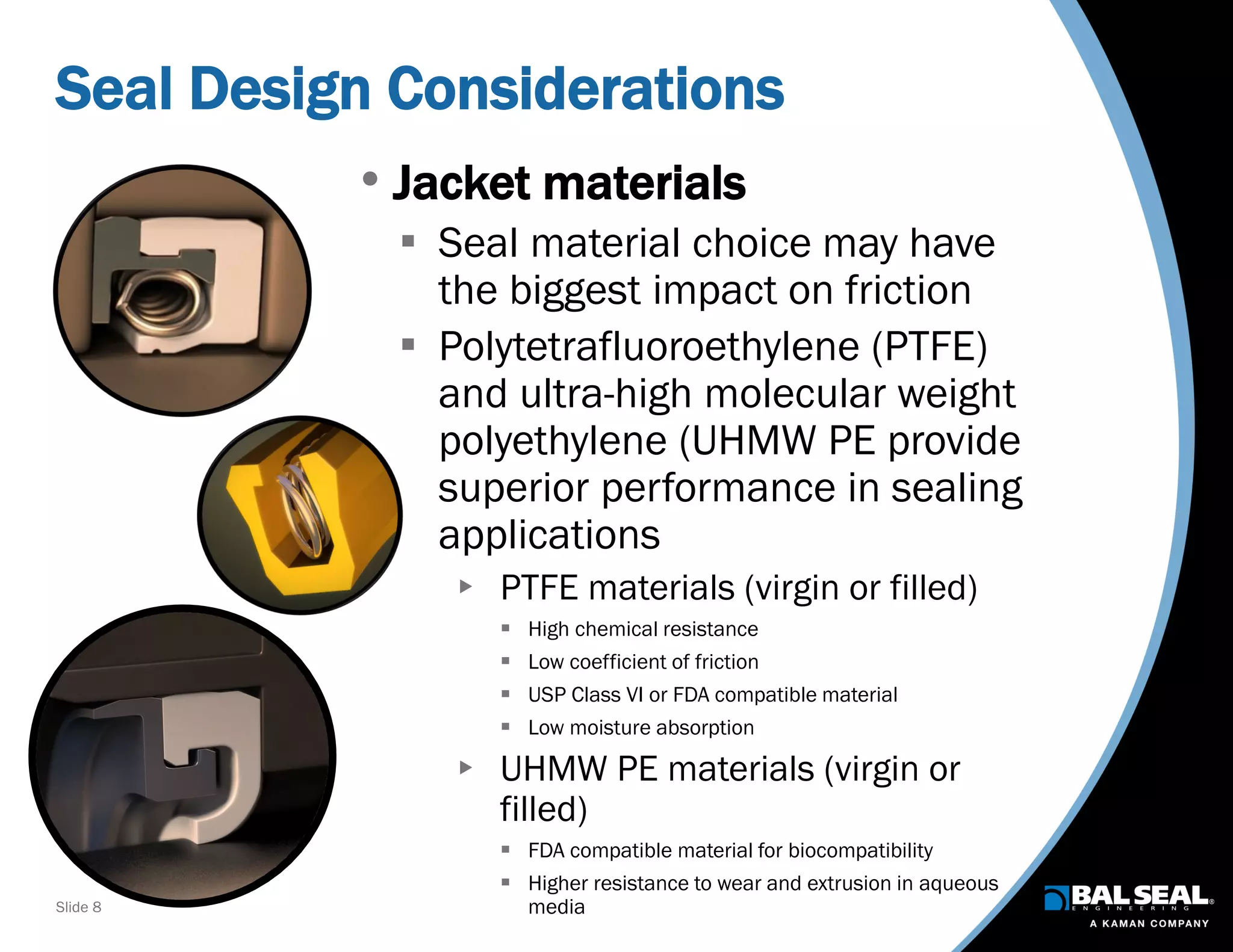
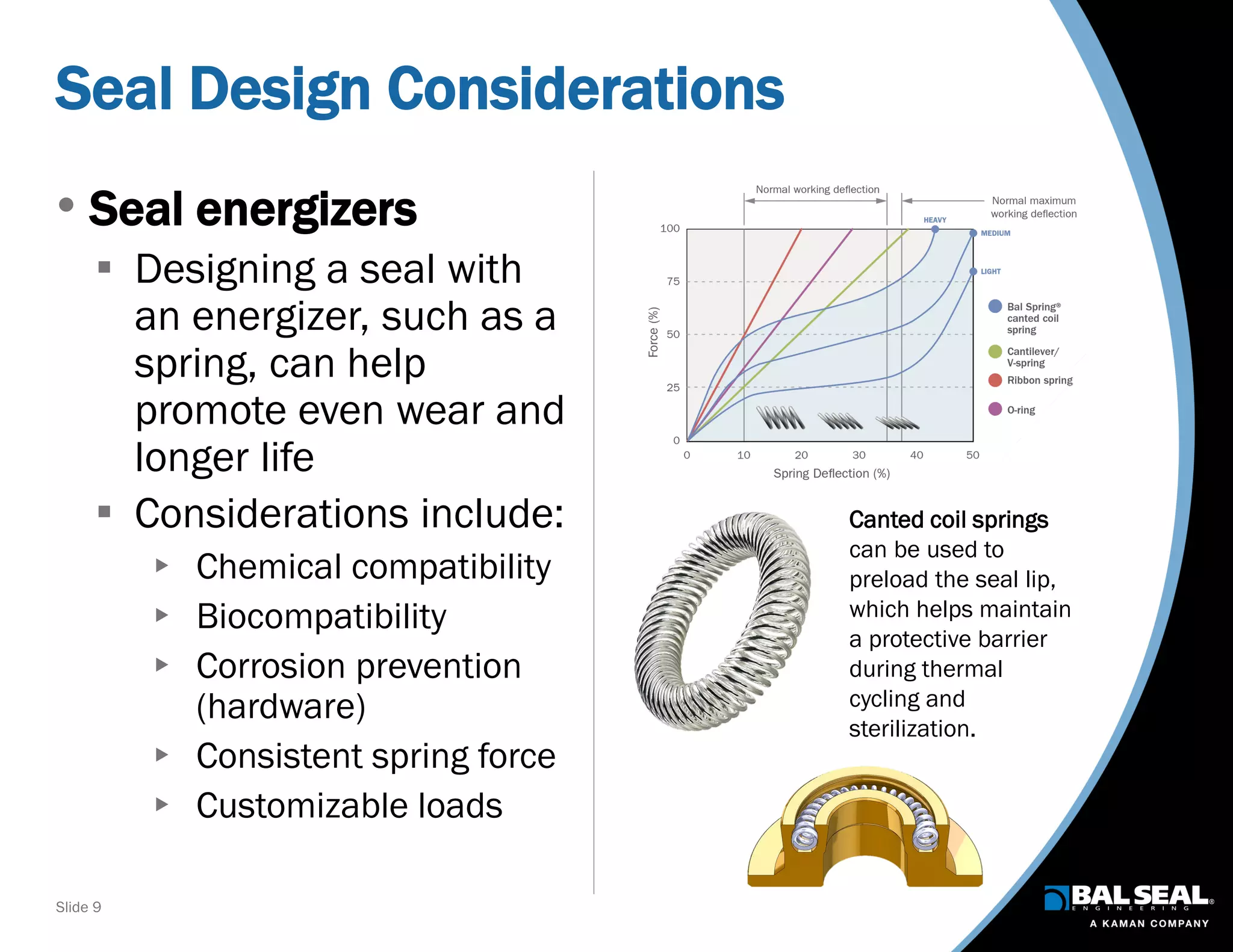
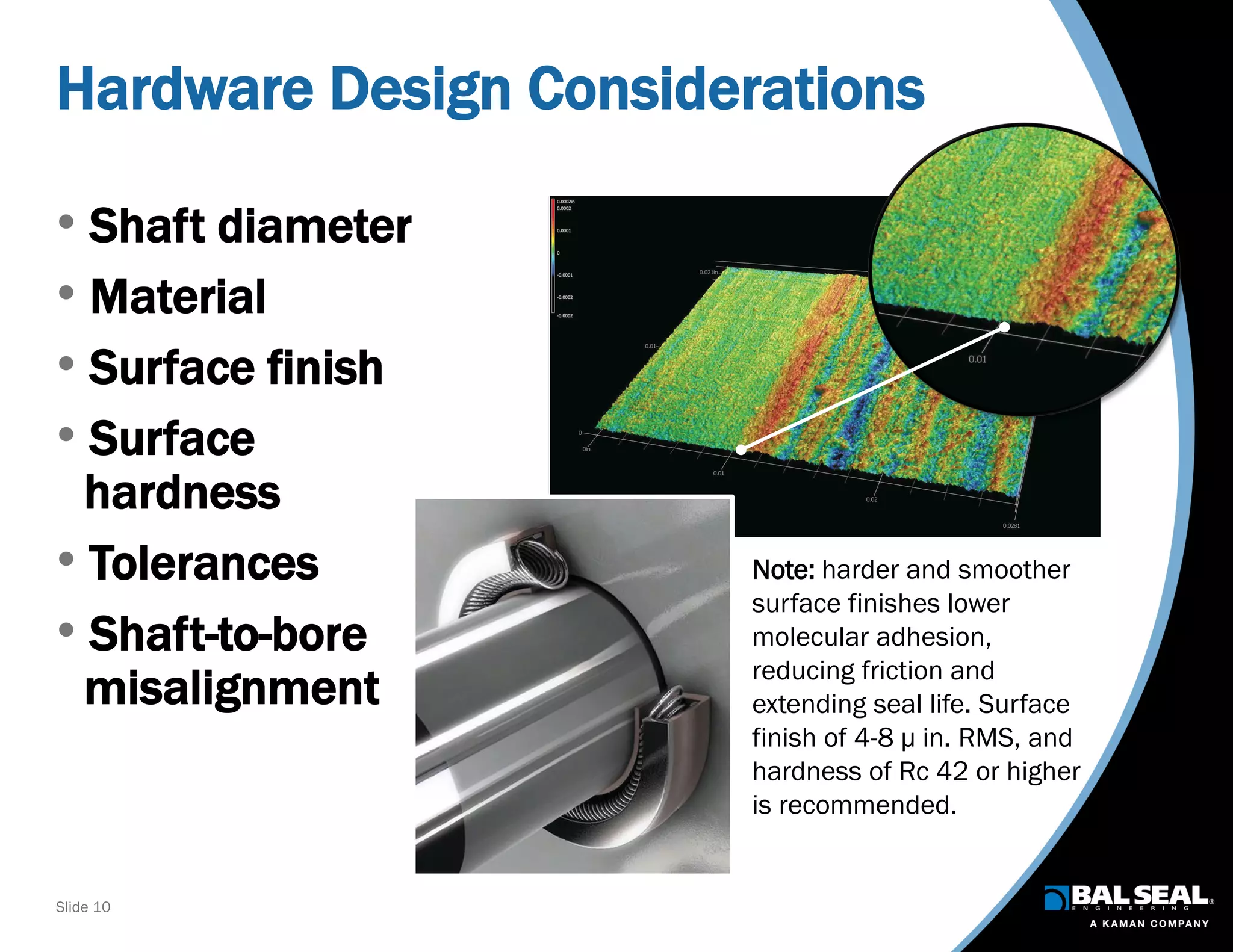
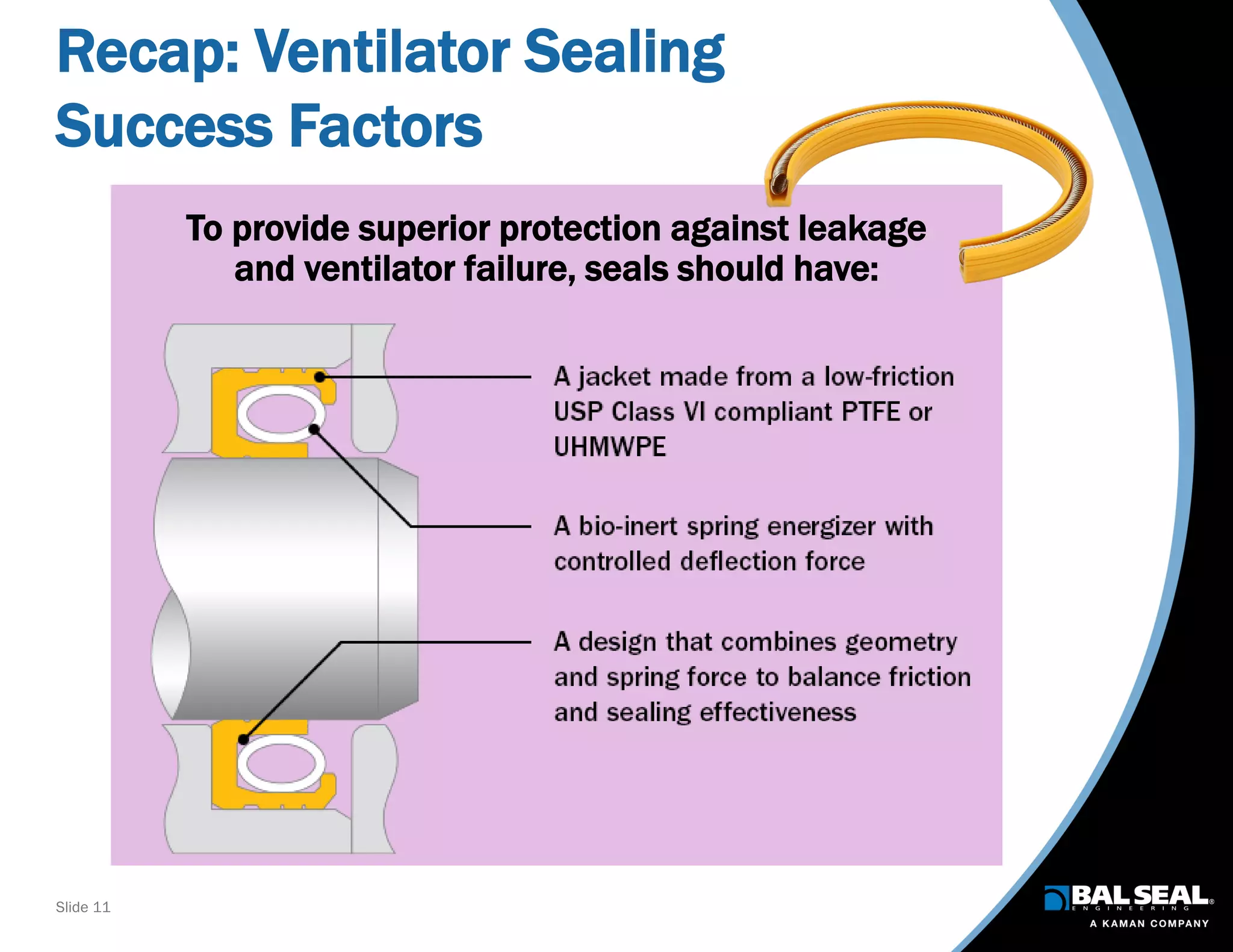
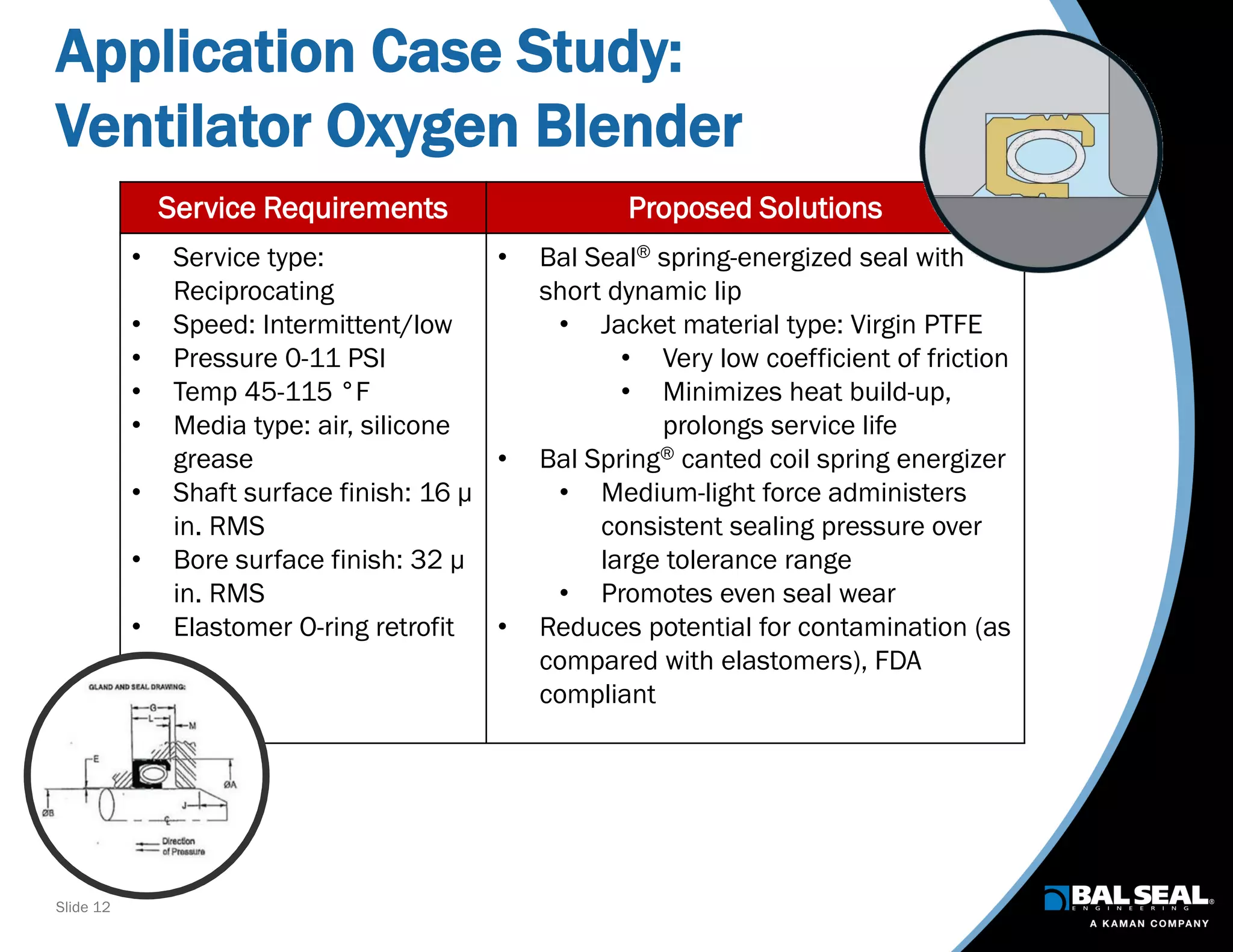
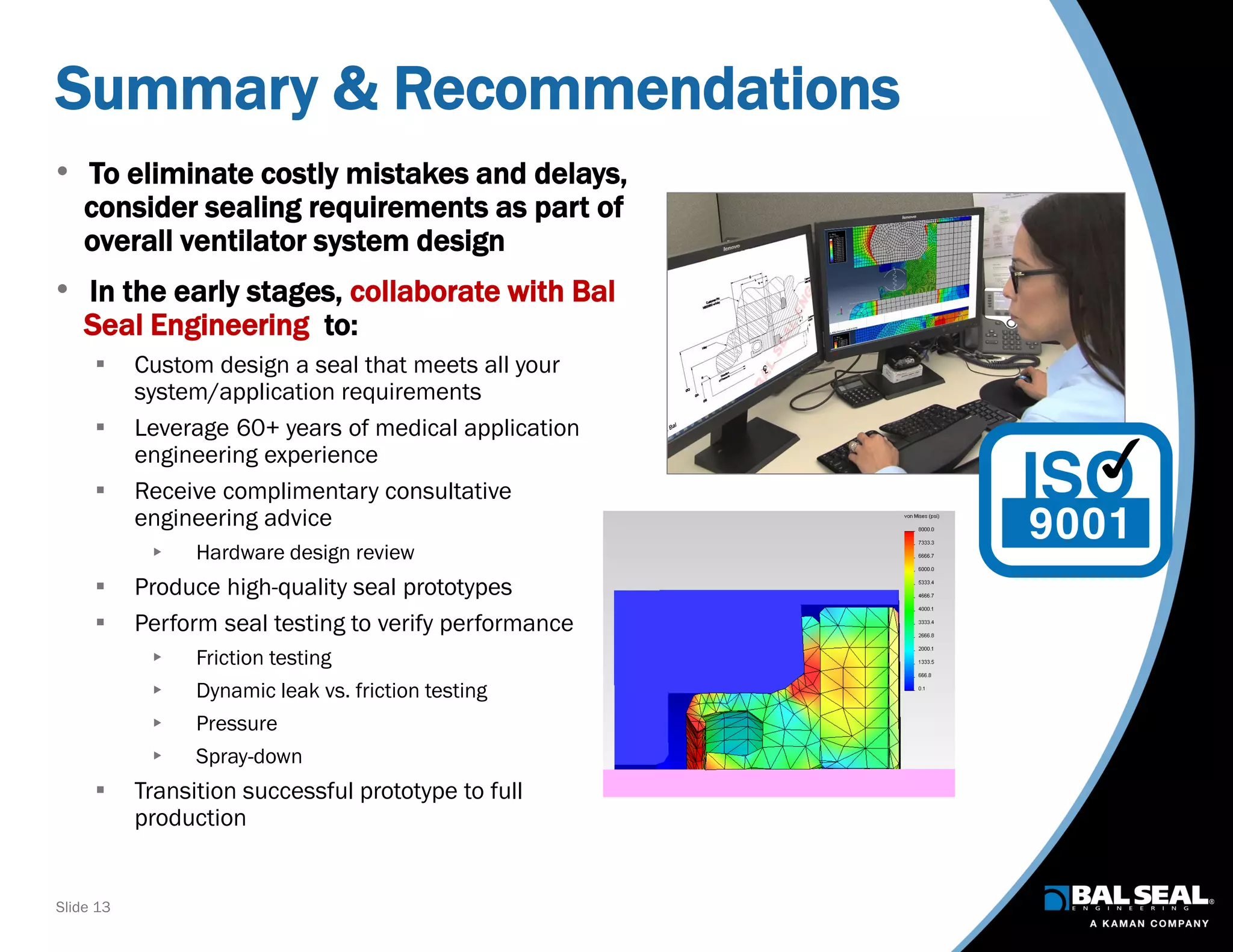
The document discusses the importance of ventilators in respiratory care, particularly during the COVID-19 pandemic, outlining various types, components, and operating requirements necessary for their effective function. It emphasizes the critical role of seals in ventilators to prevent leakage and ensure reliability, while detailing design considerations for seal materials, geometry, and energizers to enhance performance. Additionally, it recommends collaboration with Bal Seal Engineering for custom seal design and prototyping to optimize ventilator systems.