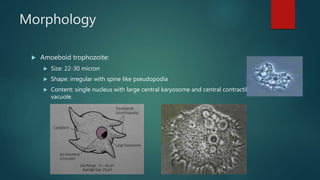
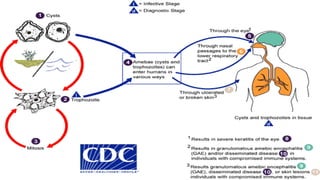
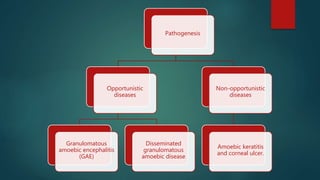
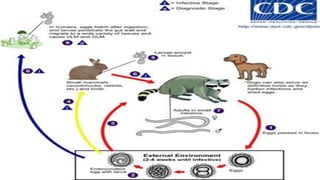
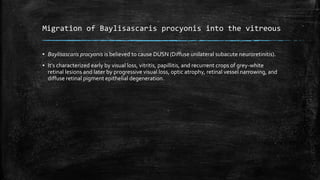
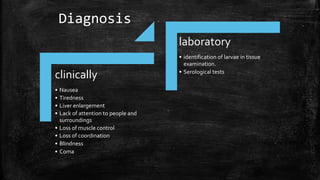
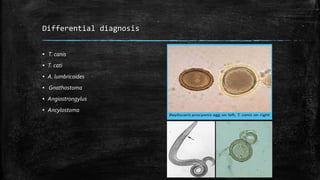
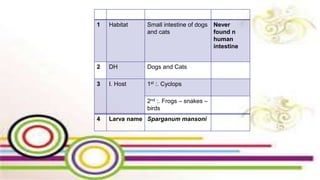
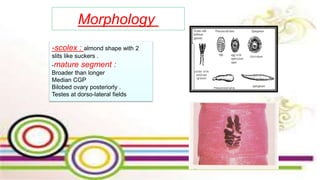
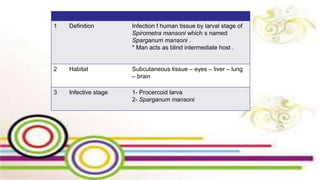
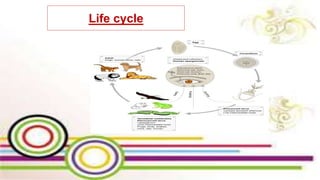
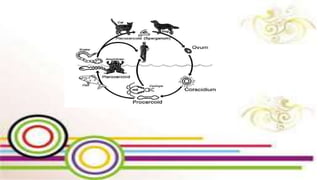
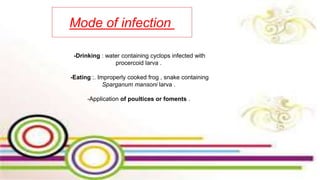
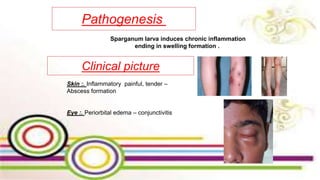
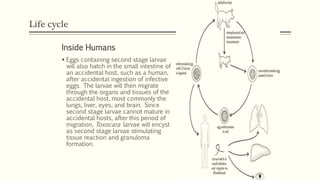
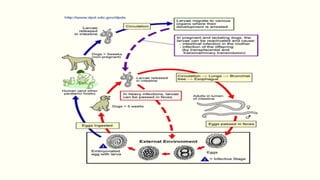
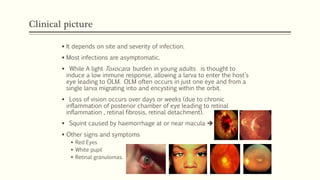
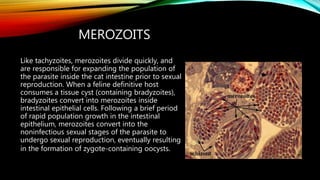
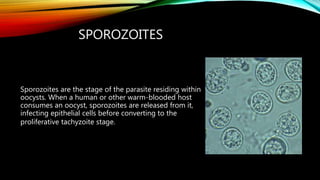
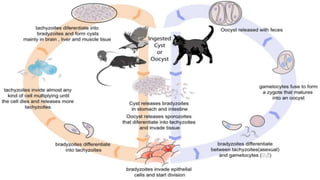
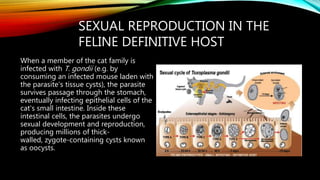
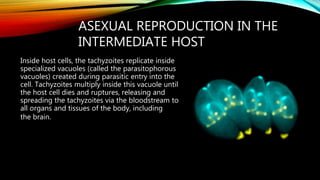
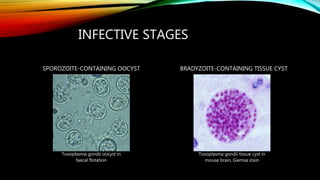
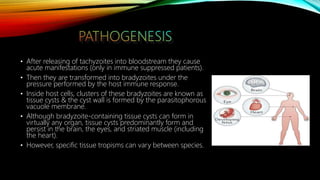
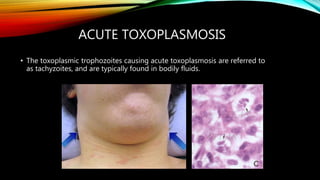
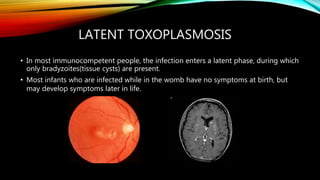
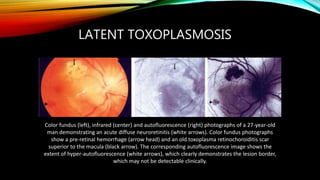
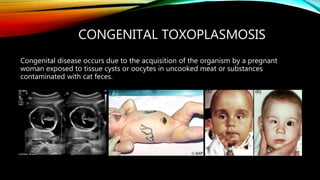
The document primarily discusses various parasitic infections caused by organisms such as Acanthamoeba spp, Baylisascaris procyonis, and Toxoplasma gondii. It provides detailed information on the morphology, life cycle, transmission, disease production, clinical manifestations, diagnosis, and treatment options associated with these parasites. Specific sections focus on the impact of these infections on human health, especially in immunocompromised individuals and those with particular risk factors, such as contact lens use.