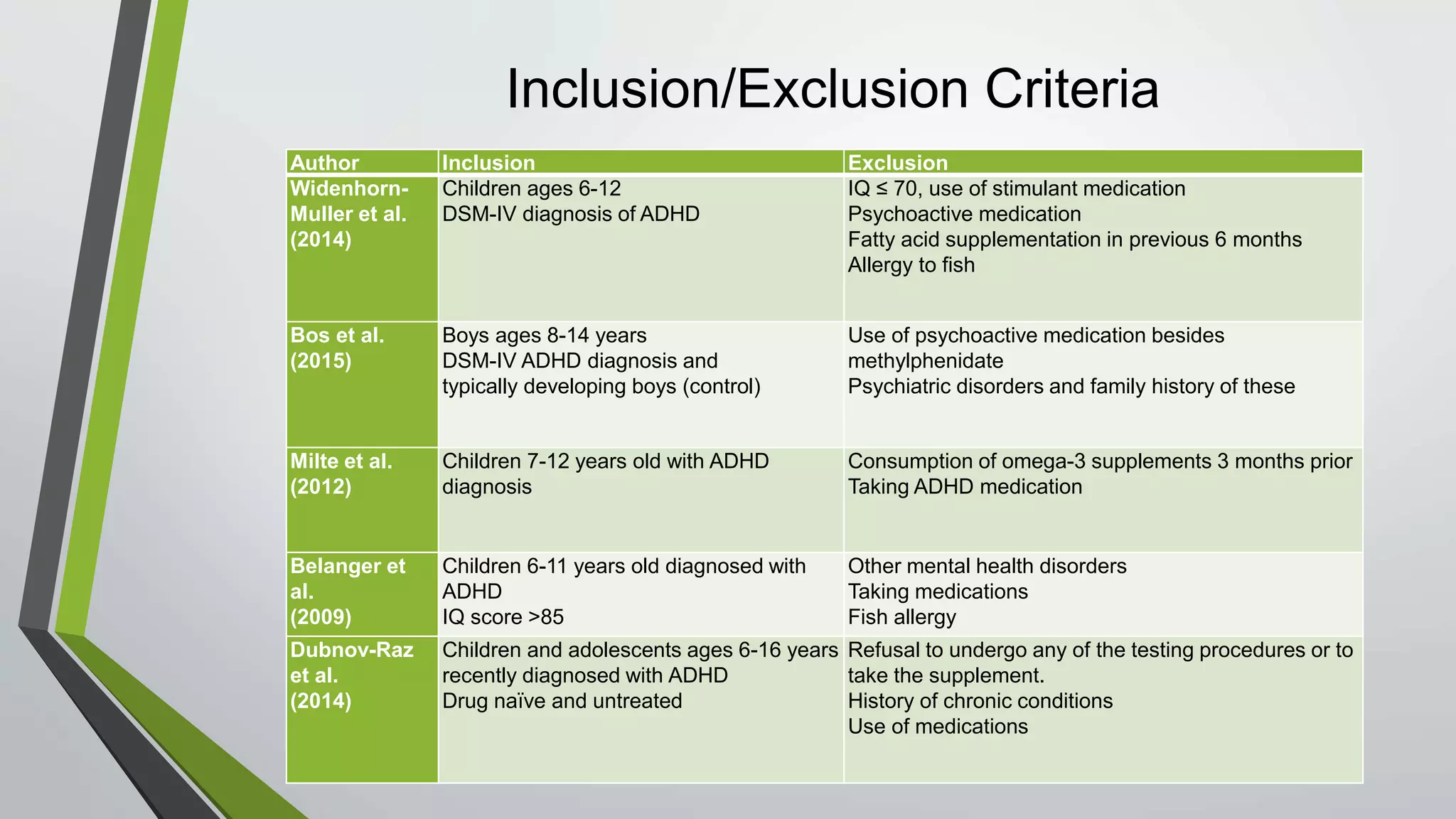
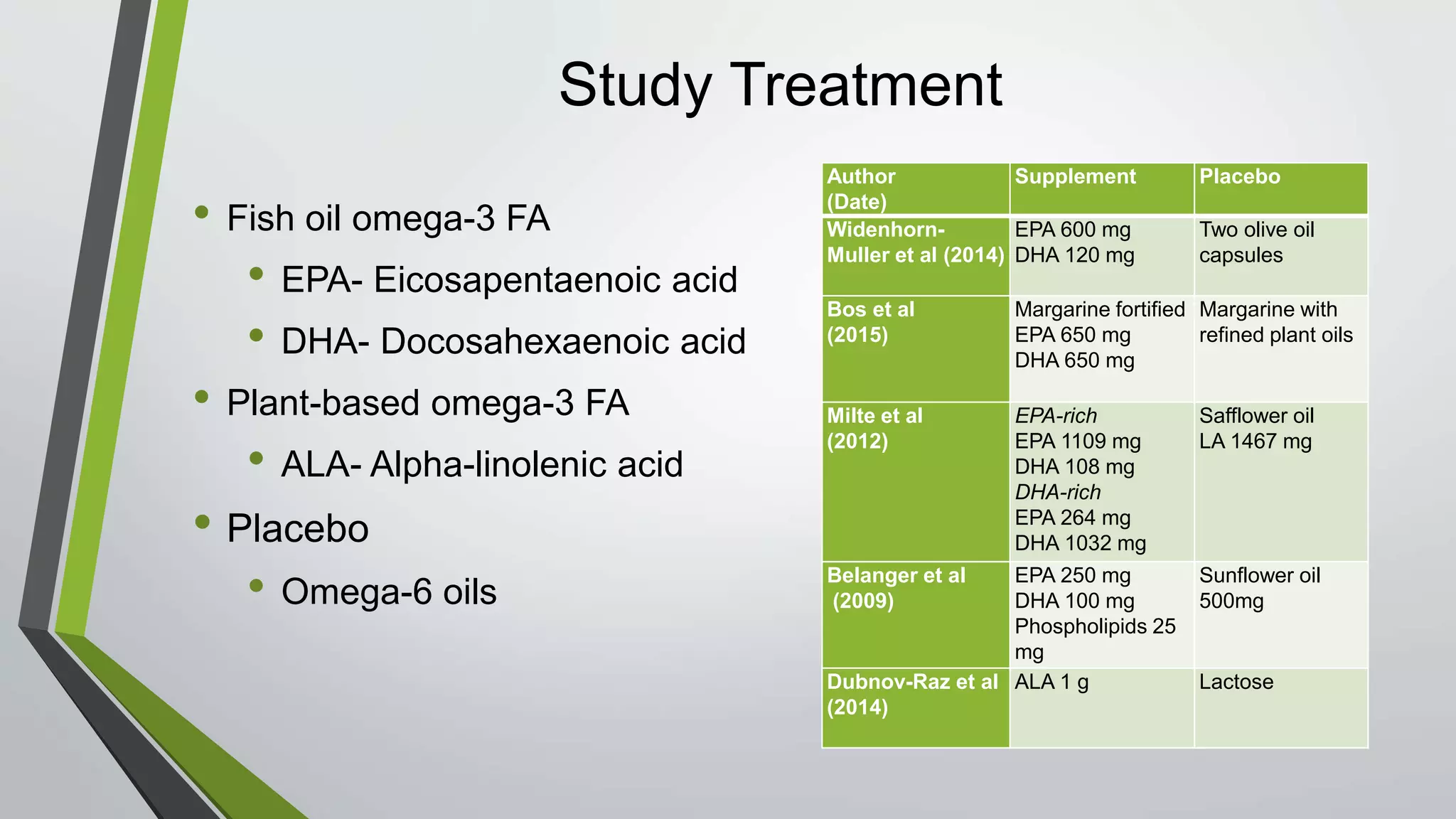
Omega-3 fatty acid supplementation was studied in 5 clinical trials involving children with ADHD. The studies examined EPA, DHA, and ALA supplementation and their effects on ADHD symptoms using parent and teacher rating scales. The results were inconclusive, with some studies finding improvements in attention and behavior and others finding no significant effects. Larger and longer studies are still needed to make clinical recommendations due to limitations like small sample sizes and short treatment durations in the existing studies.















![References
1. Kevin R Krull P. Attention deficit hyperactivity disorder in children and adolescents: Epidemiology and pathogenesis.
http://www.uptodate.com.proxy.campbell.edu/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-epidemiology-and-
pathogenesis?source=search_result&search=adhd&selectedTitle=5~150. Updated 2015. Accessed 02/12, 2016.
2. Centers for Disease Control and Prevention. Attention-deficit/ hyperactivity disorder (ADHD): Data and statistics.
http://www.cdc.gov/ncbddd/adhd/data.html. Updated 2016. Accessed 2/19, 2016.
3. Kevin R Krull P. Attention deficit hyperactivity disorder in children and adolescents: Clinical features and evaluation.
http://www.uptodate.com.proxy.campbell.edu/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-clinical-features-and-
evaluation?source=search_result&search=adhd+children&selectedTitle=2~150. Updated 2015. Accessed 2/16, 2016.
4. Widenhorn-Muller K, Schwanda S, Scholz E, Spitzer M, Bode H. Effect of supplementation with long-chain omega-3 polyunsaturated fatty
acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention
trial. Prostaglandins Leukot Essent Fatty Acids. 2014;91(1-2):49-60. doi: 10.1016/j.plefa.2014.04.004 [doi].
5. Bos DJ, Oranje B, Veerhoek ES, et al. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and
without attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2015;40(10):2298-2306. doi: 10.1038/npp.2015.73 [doi].
6. Dubnov-Raz G, Khoury Z, Wright I, Raz R, Berger I. The effect of alpha-linolenic acid supplementation on ADHD symptoms in children: A
randomized controlled double-blind study. Front Hum Neurosci. 2014;8:780. doi: 10.3389/fnhum.2014.00780 [doi].
7. Belanger SA, Vanasse M, Spahis S, et al. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A
randomized, double-blind, placebo-controlled study. Paediatr Child Health. 2009;14(2):89-98.
8. Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PR. Increased erythrocyte eicosapentaenoic acid and docosahexaenoic
acid are associated with improved attention and behavior in children with ADHD in a randomized controlled three-way crossover trial. J Atten
Disord. 2015;19(11):954-964. doi: 10.1177/1087054713510562 [doi].](https://image.slidesharecdn.com/0d111c68-74c3-40c9-9338-69dd445258b0-160424200412/75/Omega-3-Fatty-Acid-Supplementation-in-Children-with-ADHD-19-2048.jpg)