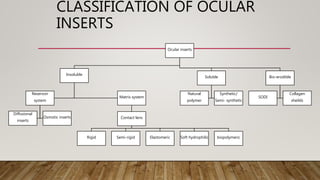
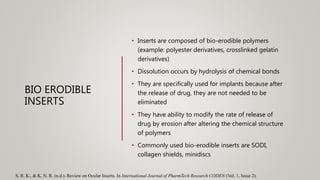
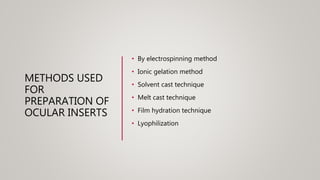
This document discusses ocular inserts, which are thin, multilayered devices placed in the eye to provide sustained release of drugs for ocular diseases. It describes how ocular inserts are made of biodegradable polymers and can achieve increased bioavailability. The document outlines different types of ocular inserts including insoluble inserts like reservoir and matrix systems, and soluble inserts made from natural or synthetic polymers. It also discusses advantages of ocular inserts like reduced systemic side effects and improved patient compliance compared to traditional eye drop delivery.