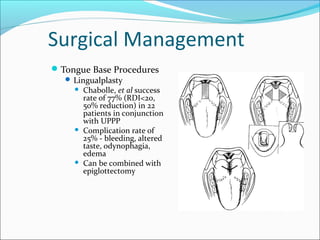
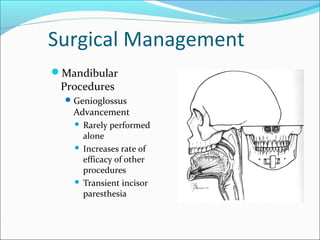
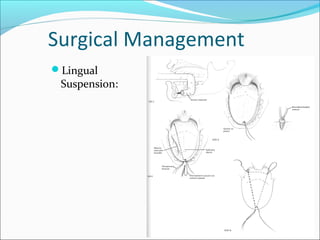
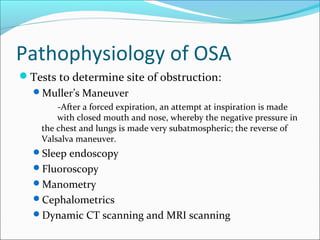
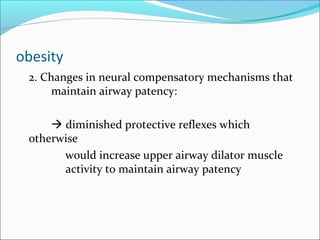
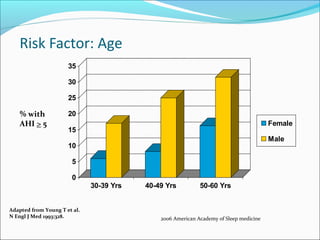
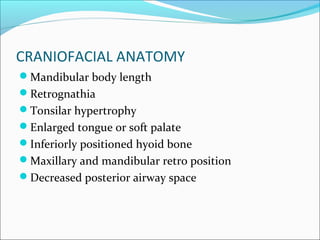
The document provides an extensive overview of sleep, detailing its stages, biological rhythms, and the importance of sleep for health. It discusses sleep disorders, particularly obstructive sleep apnea (OSA), its prevalence, risk factors, symptoms, and diagnosis methods, as well as available treatments including behavioral changes, medical therapies, and surgical options. Additionally, it highlights the cardiovascular risks associated with OSA and the impact of sleep disorders on overall well-being.








































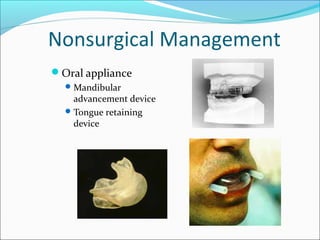
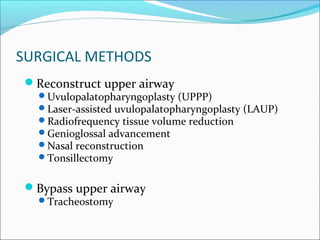
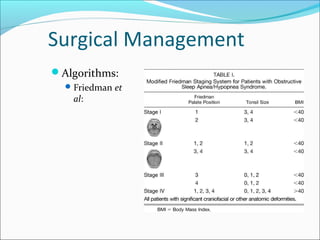
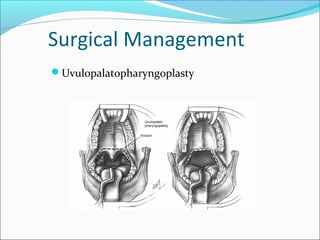
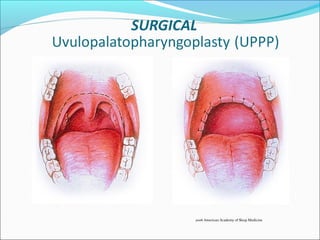
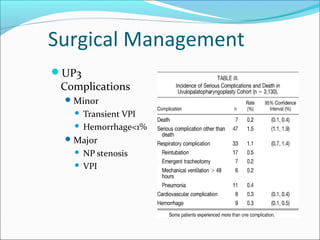
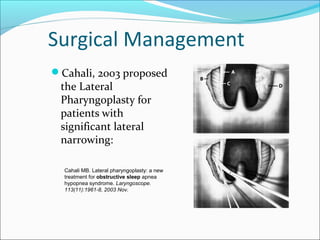
![Surgical Management
Tracheostomy
Primary treatment modality
Once placed, uncommon to decannulate
Thatcher GW. Maisel RH. The long-term evaluation of tracheostomy in the
management of severe obstructive sleep apnea. [Journal Article] Laryngoscope.
113(2):201-4, 2003 Feb.](https://image.slidesharecdn.com/10osa-161102133800/85/OBSTRUCTIVE-SLEEP-APNEA-76-320.jpg)