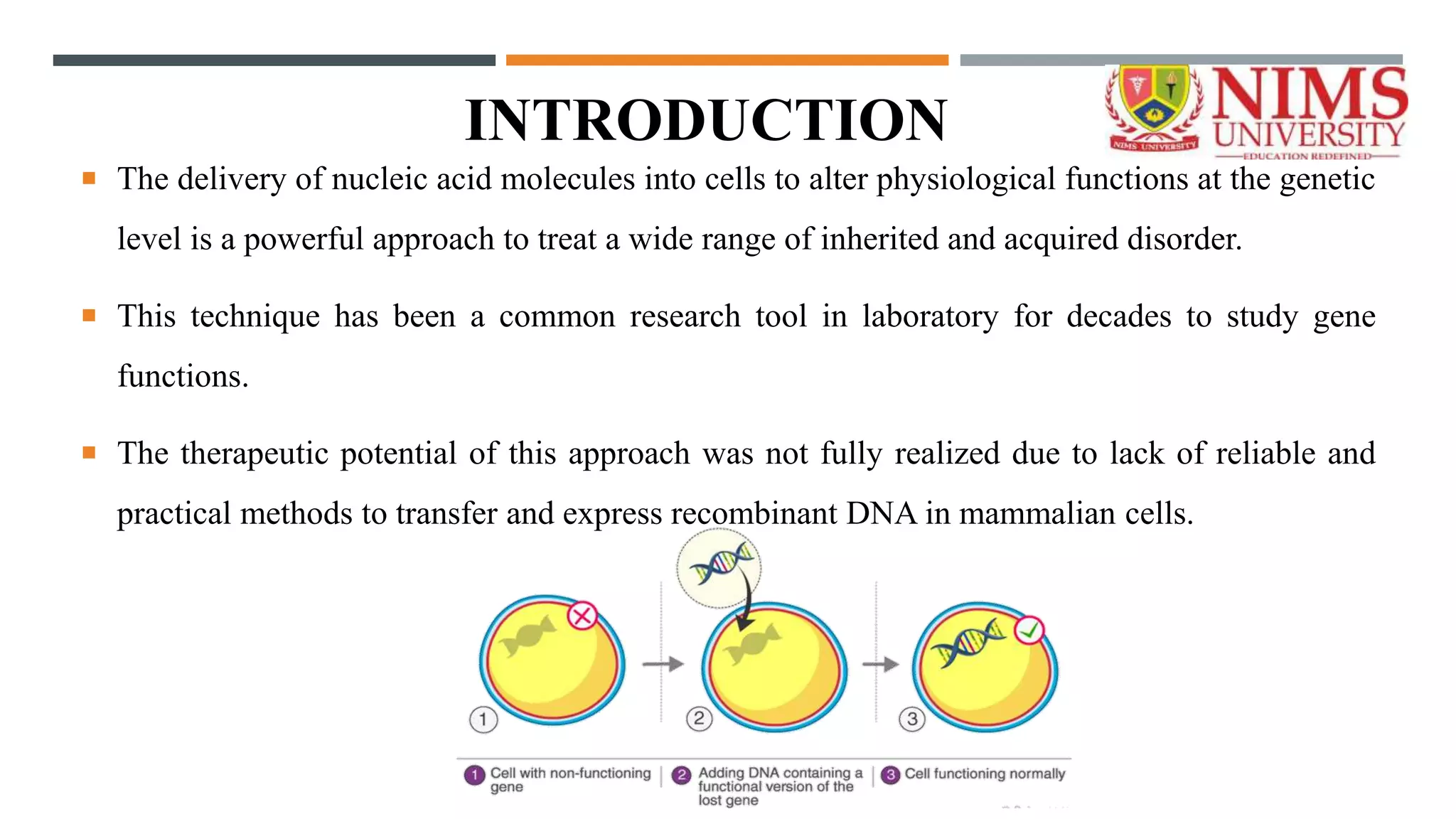
The document outlines a nucleic acid-based therapeutic delivery system focusing on gene therapy's potential to treat various genetic disorders by introducing therapeutic genes into cells. It discusses two main types of gene therapy: germline and somatic, detailing their processes and vector systems, including viral and non-viral methods. Additionally, it highlights the limitations of gene therapy and recent advancements, including FDA approvals and ongoing research in the field.