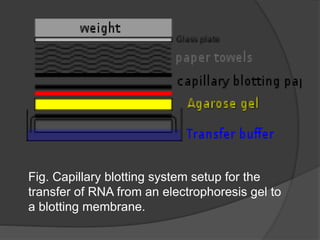
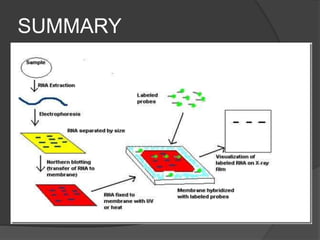
Northern blotting is a technique used to detect specific RNA sequences in a sample. It involves isolating RNA, separating it via gel electrophoresis, transferring it to a membrane, then hybridizing probes with complementary sequences to the target RNA. This allows visualization of gene expression patterns between tissues, developmental stages, and disease states from the detected RNA sequences.