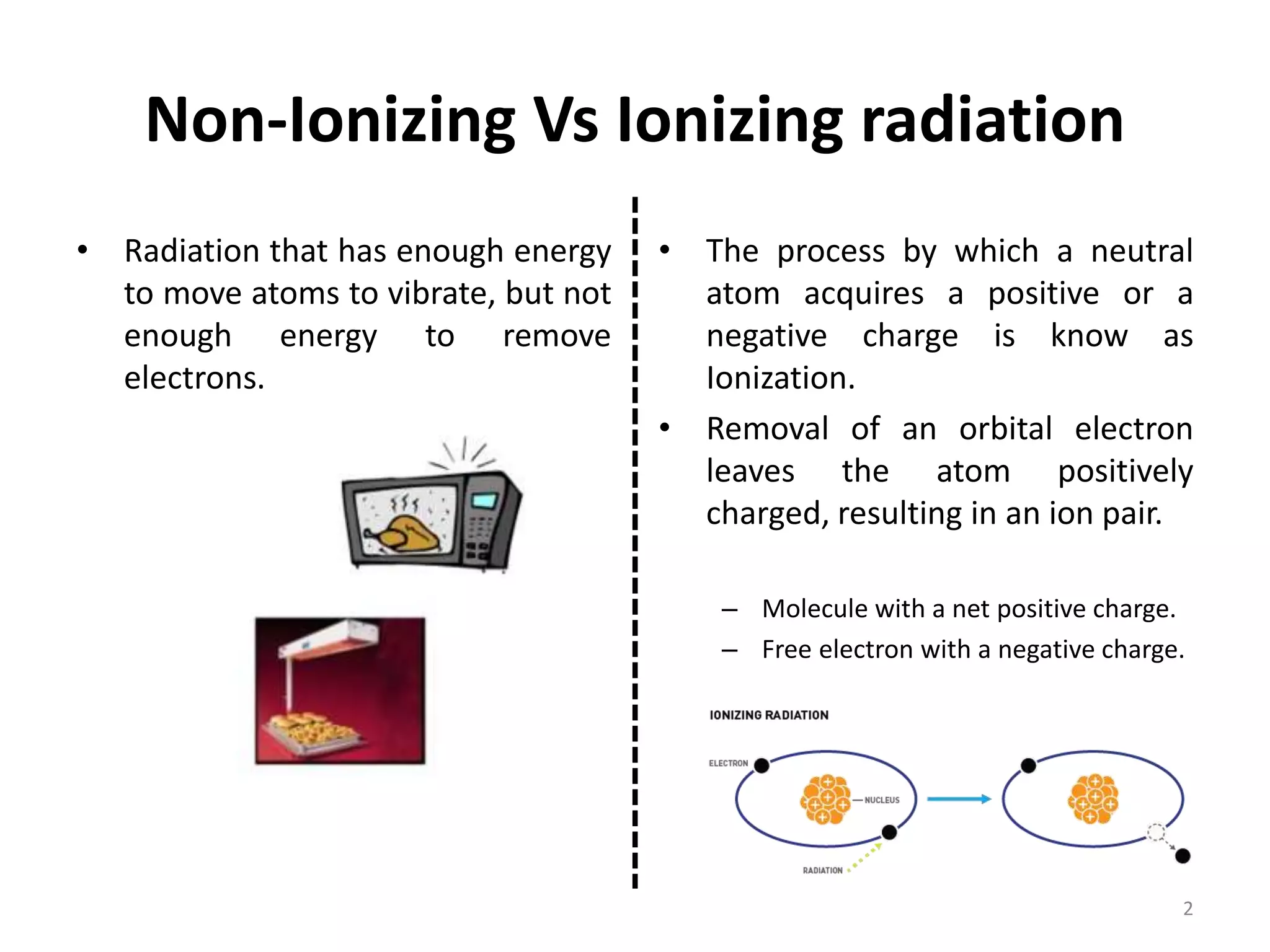
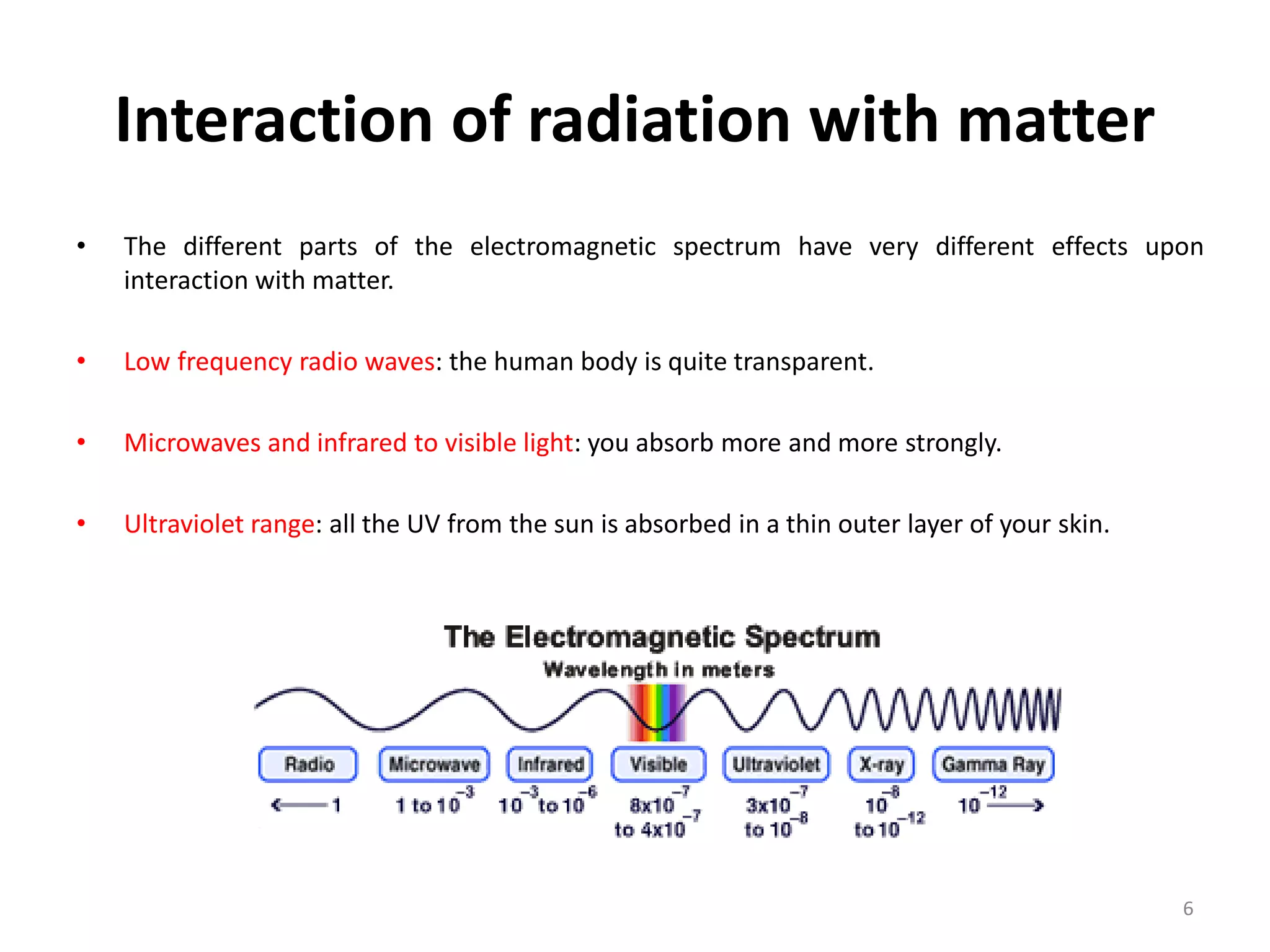
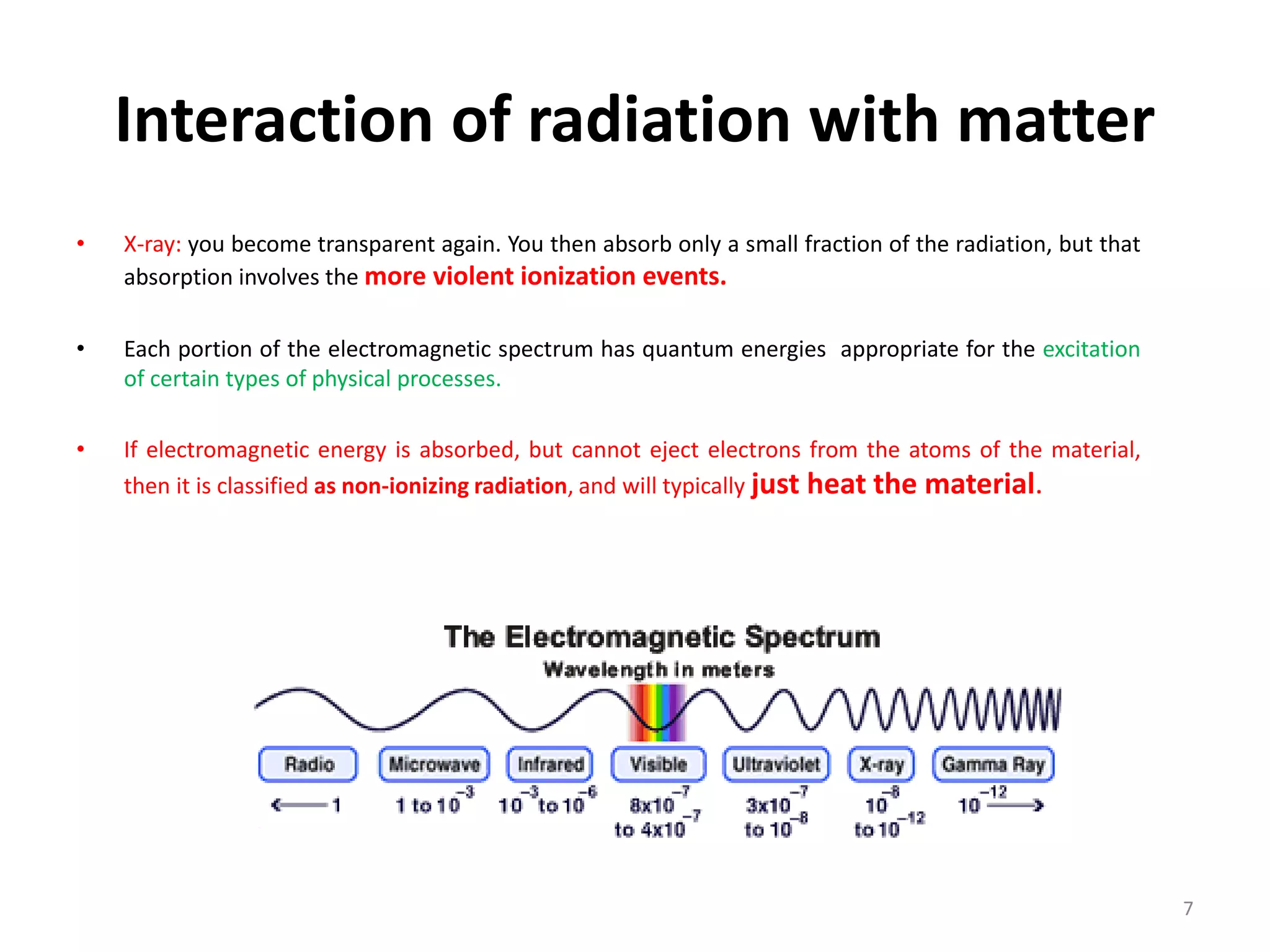
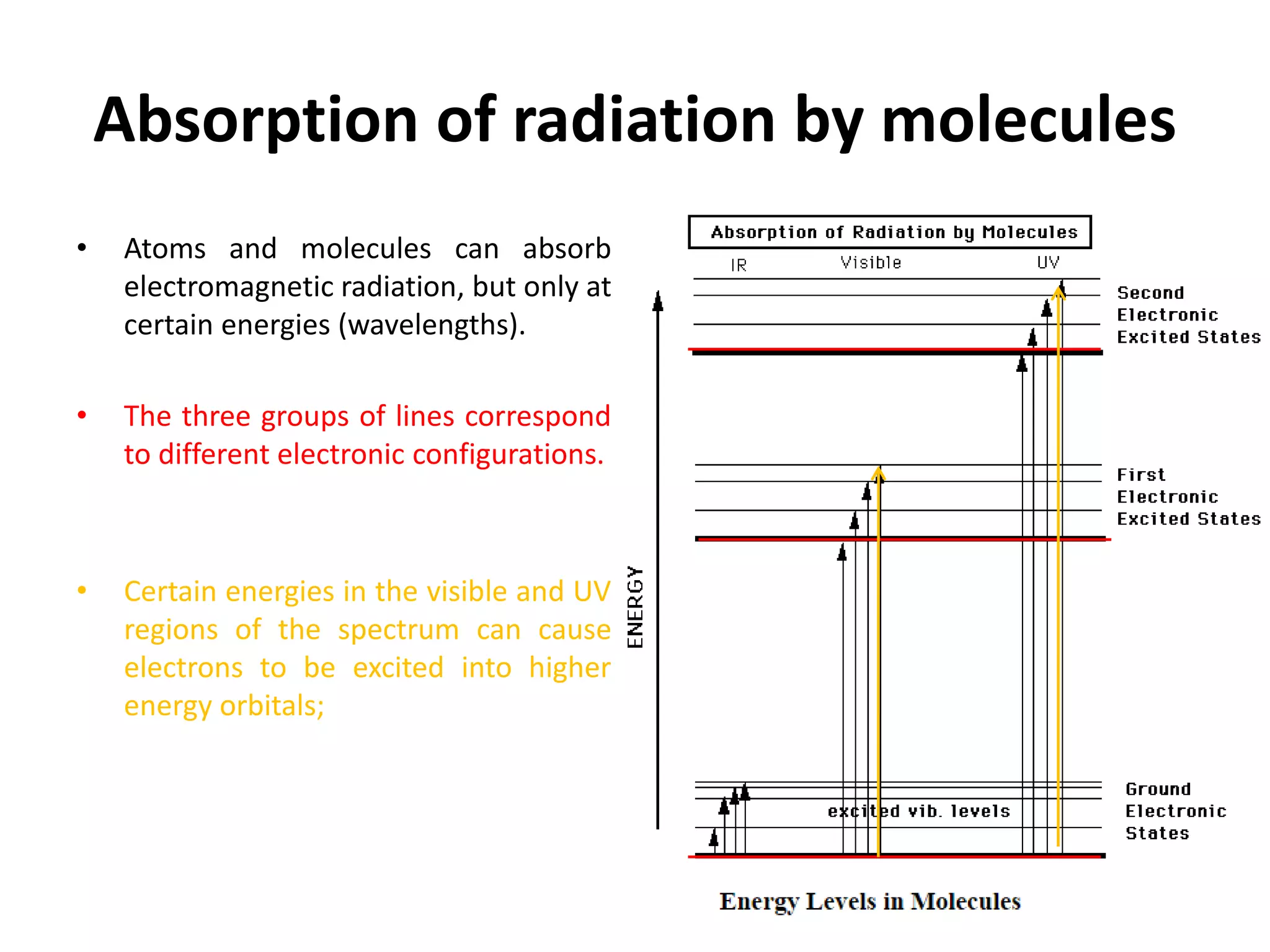
Non-ionizing radiation includes electromagnetic radiation such as radio waves, microwaves, infrared, visible light, and ultraviolet radiation. This radiation does not have enough energy to ionize atoms or molecules, but can still be absorbed, causing excitation of electrons or increasing molecular motion and vibration. Ionizing radiation like X-rays and gamma rays have high enough energies to remove electrons from atoms, causing ionization which can damage biological tissues. The distinction between ionizing and non-ionizing radiation is important as ionizing radiation can directly cause mutation or cancer.