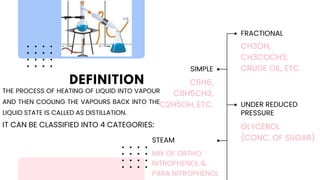
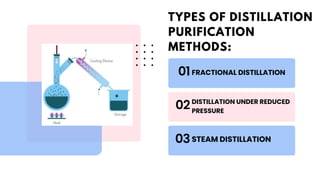
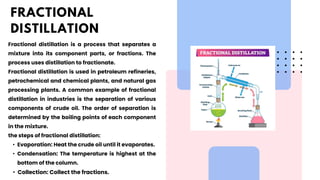
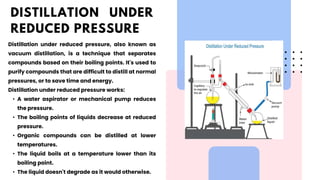
The document discusses various methods of purification in chemistry, focusing specifically on distillation techniques. It outlines three main types: fractional distillation, distillation under reduced pressure, and steam distillation, detailing their processes and applications. Acknowledgments are made to contributors, resources, and supportive figures throughout the project.