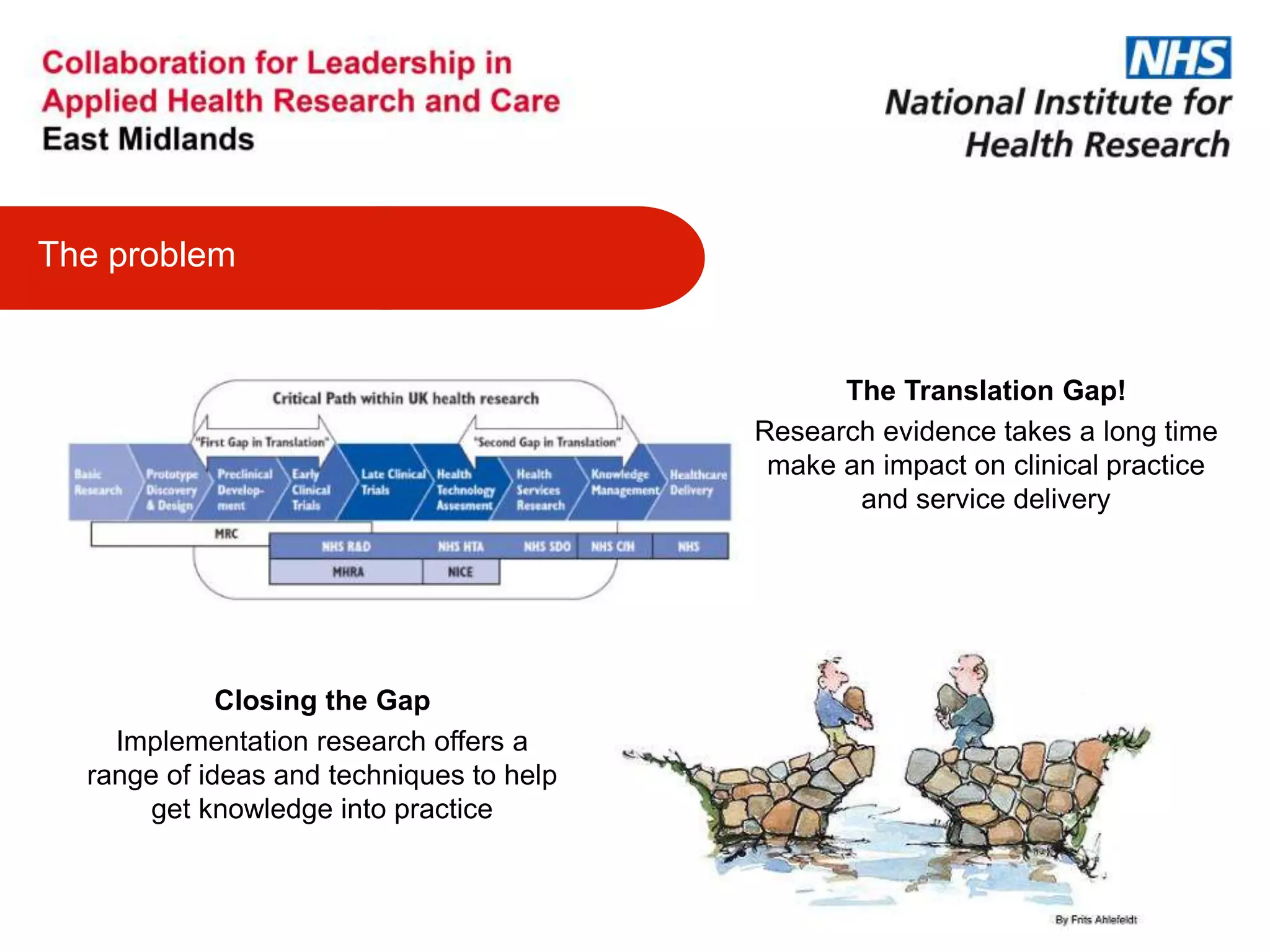
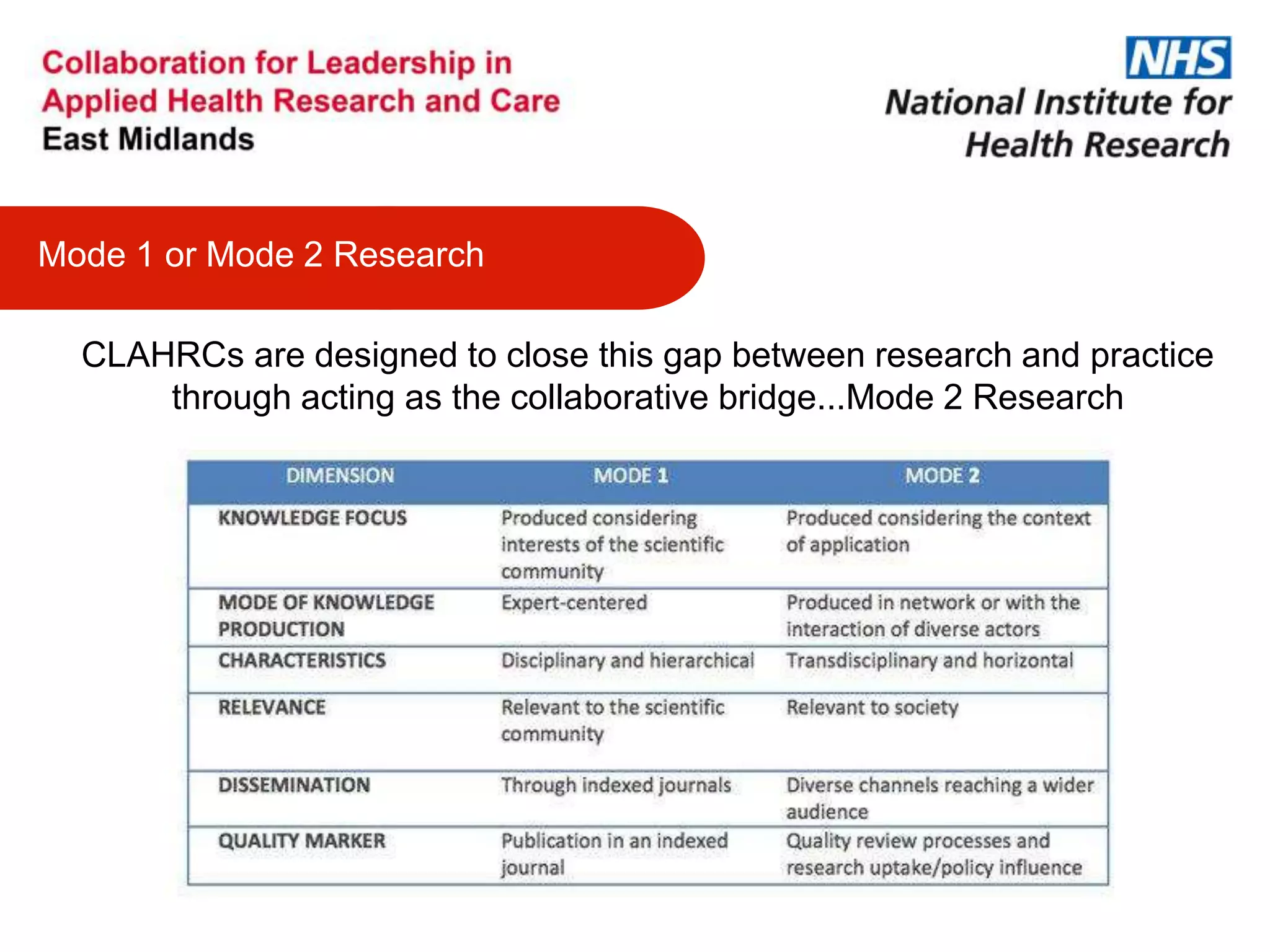
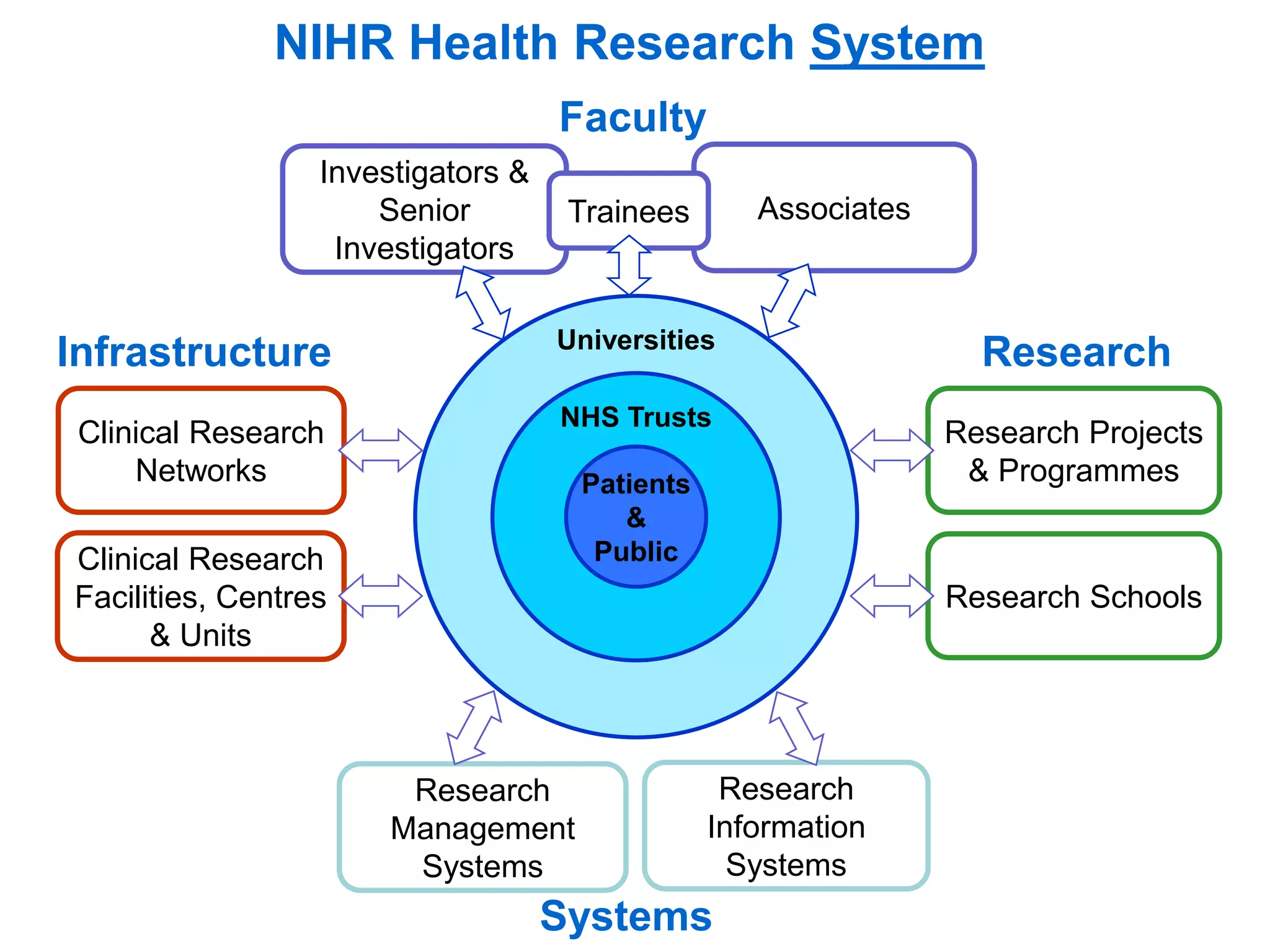
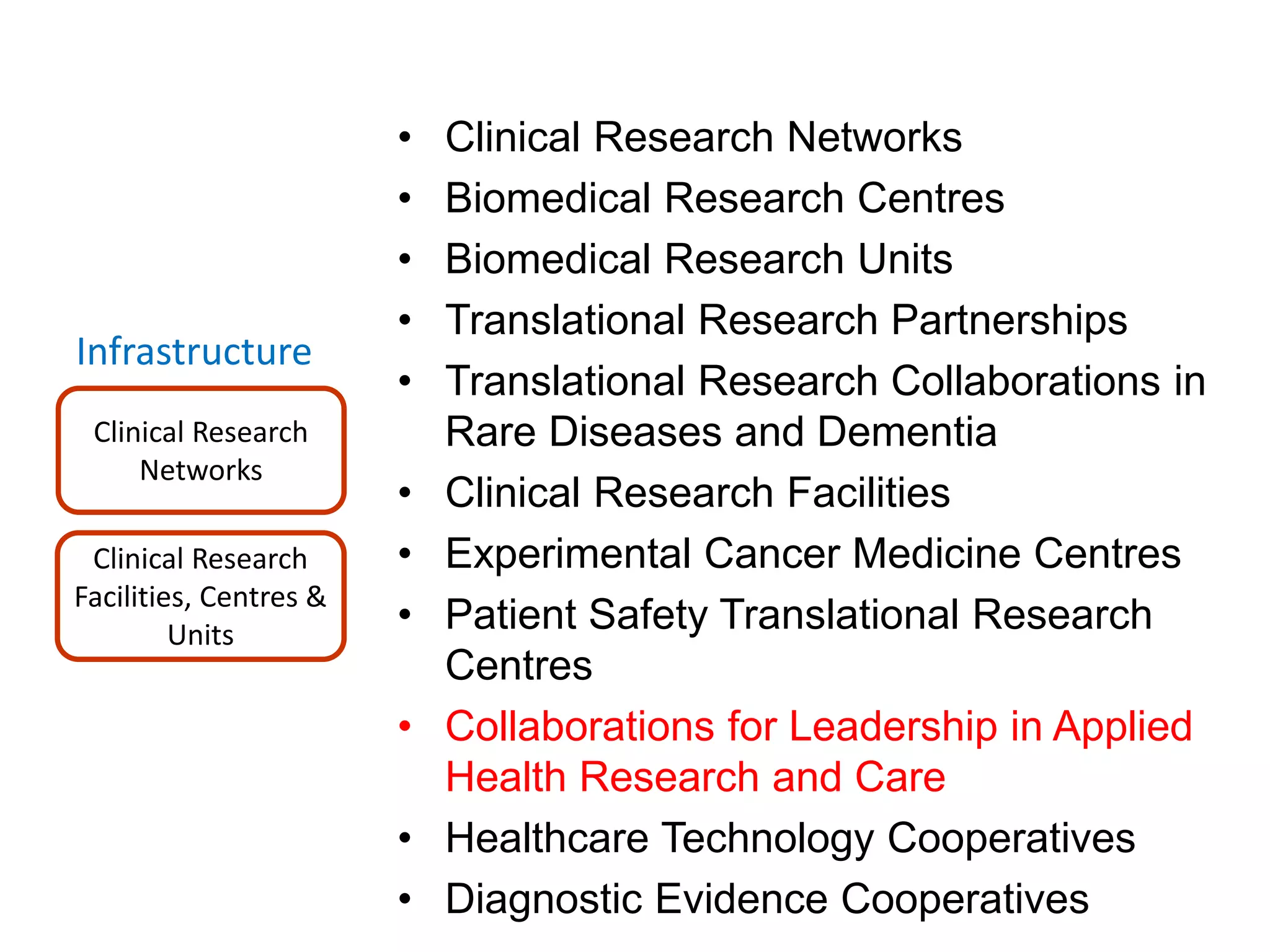
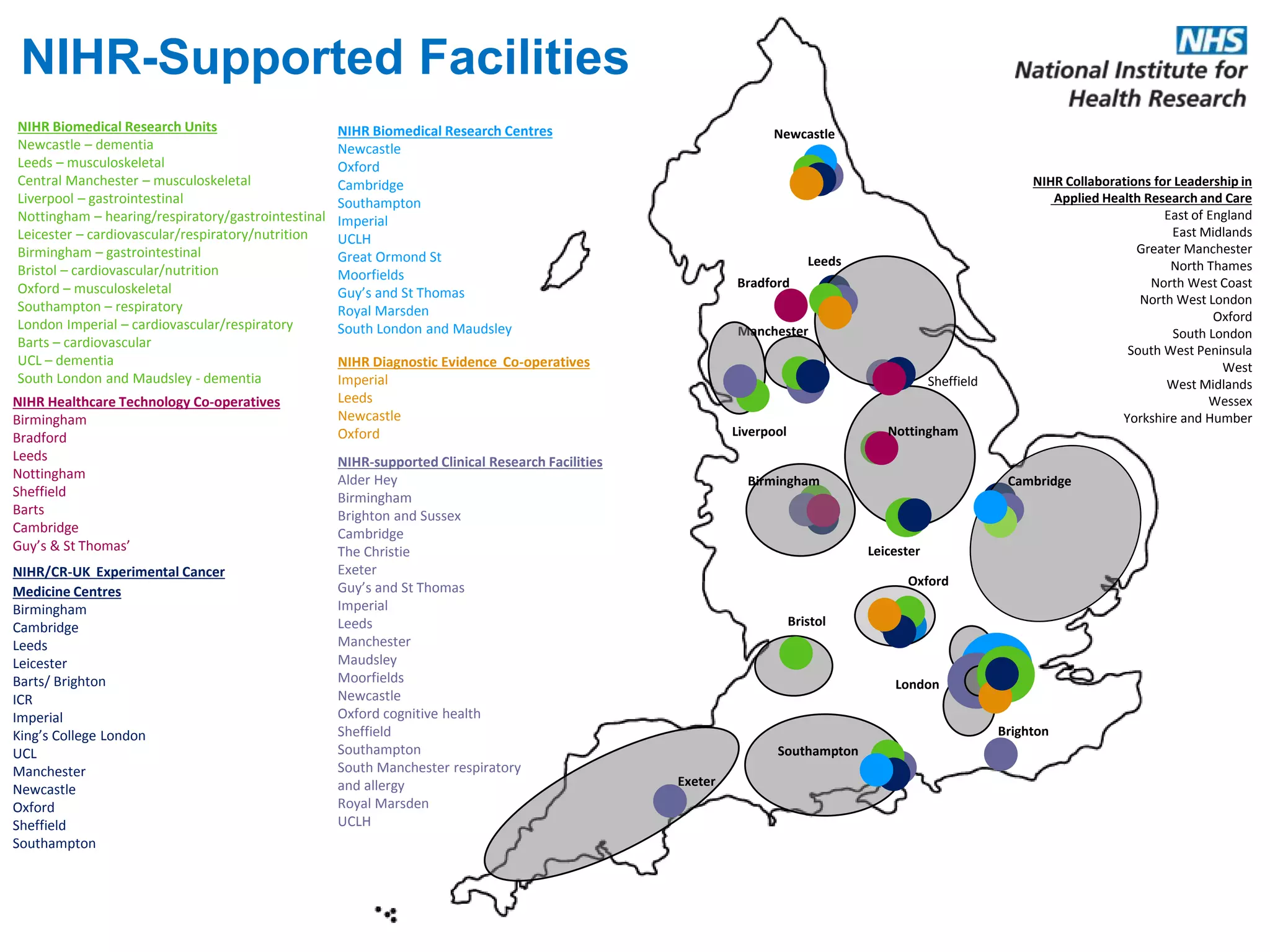
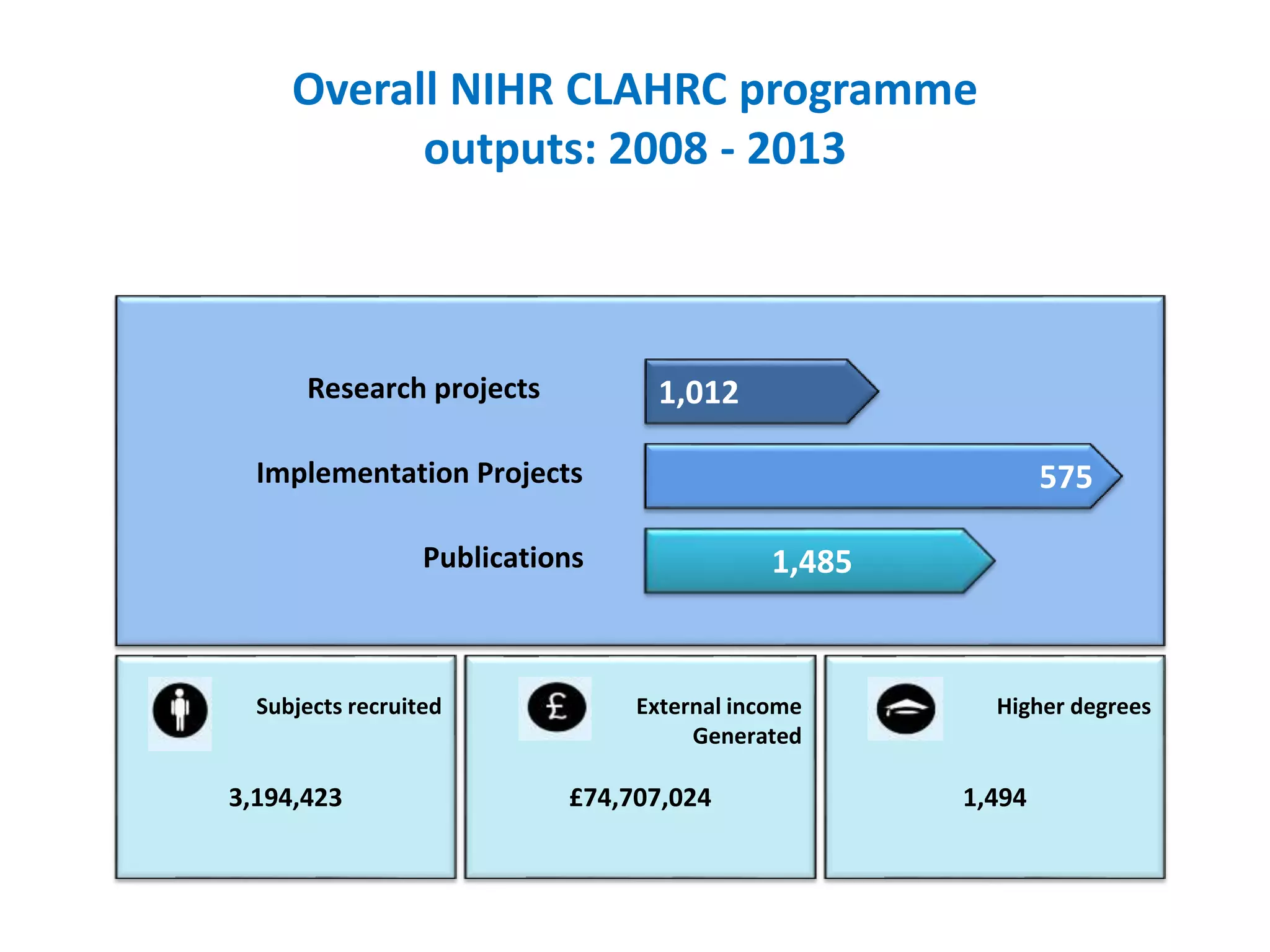
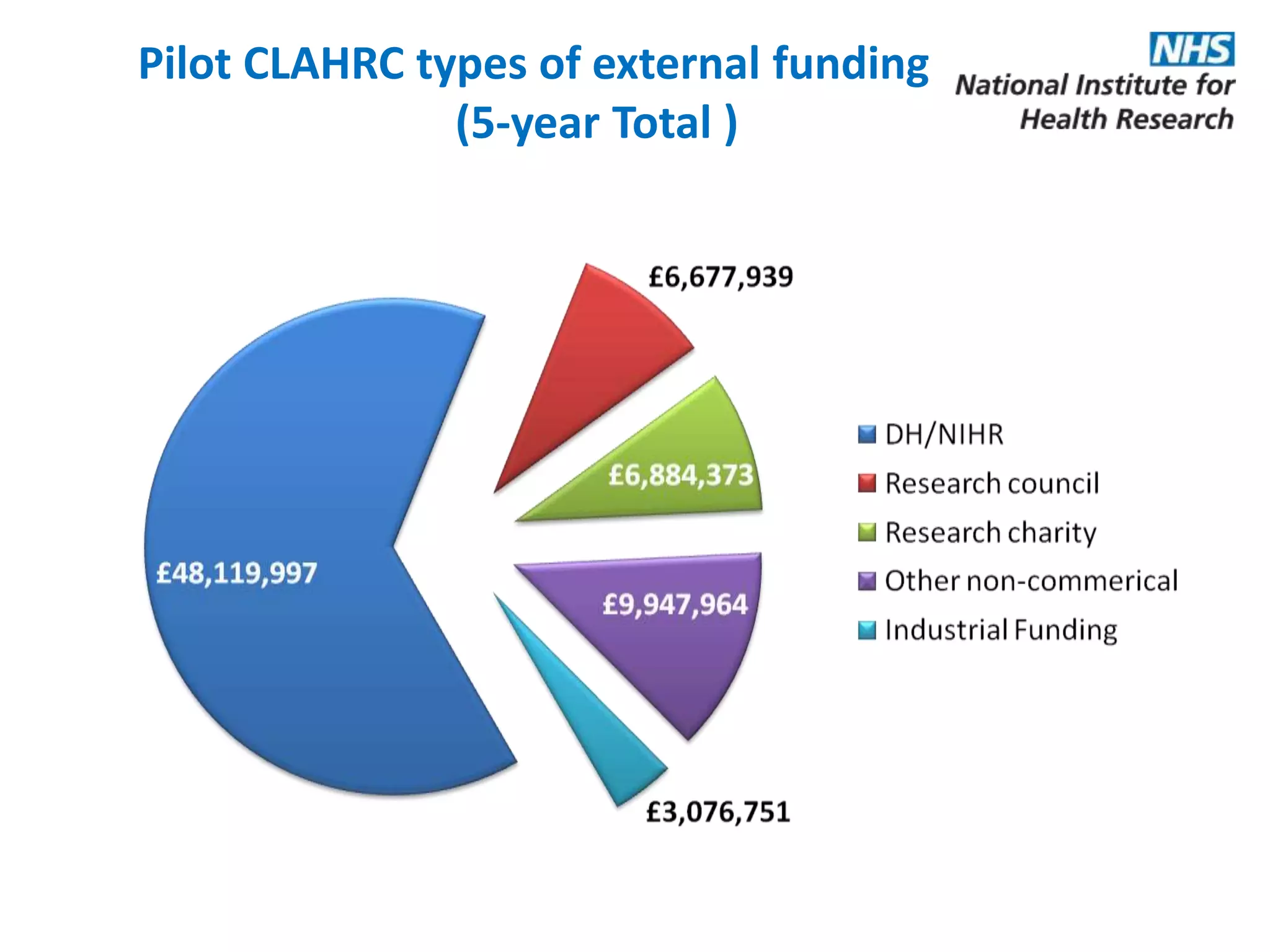
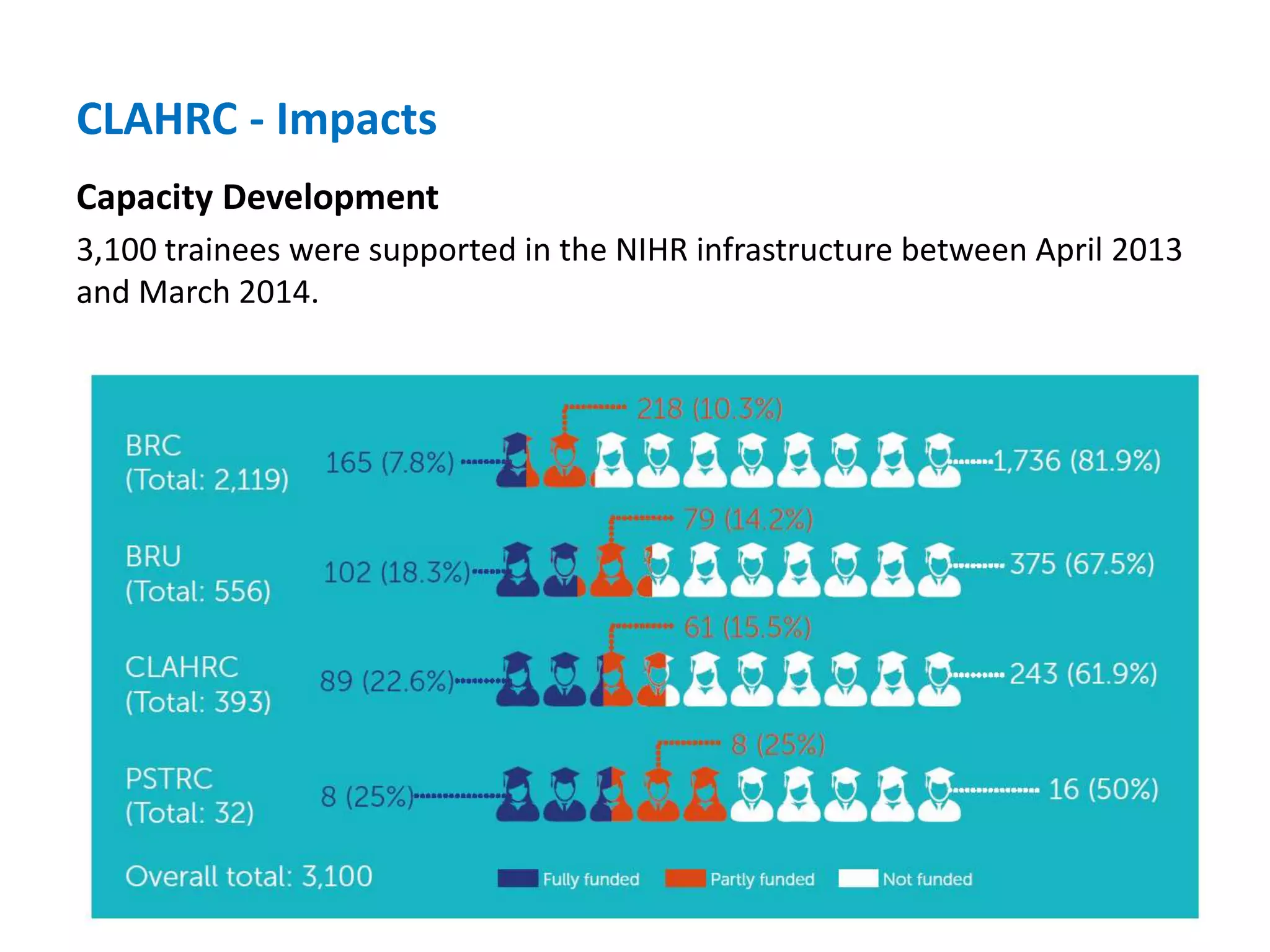
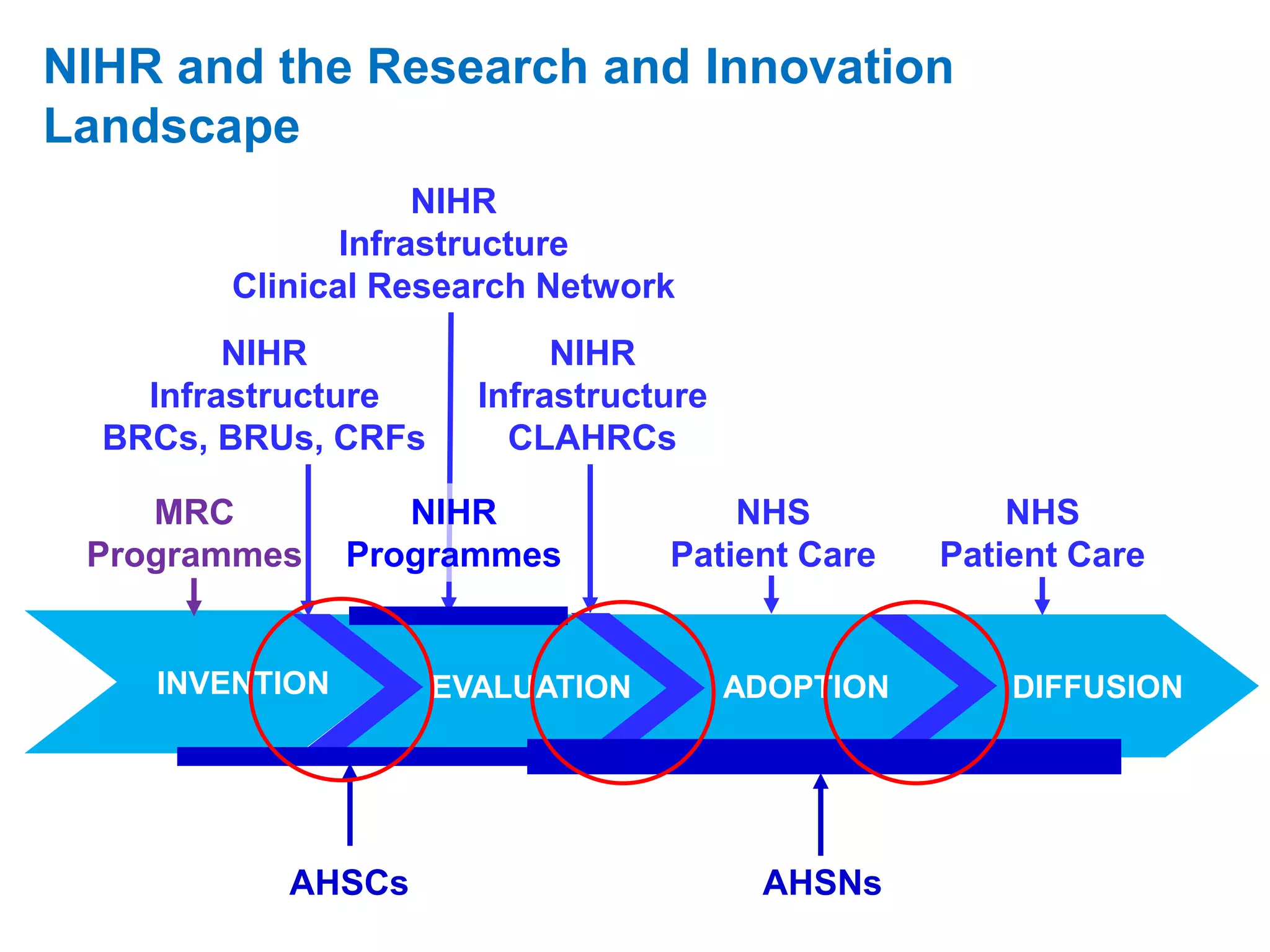
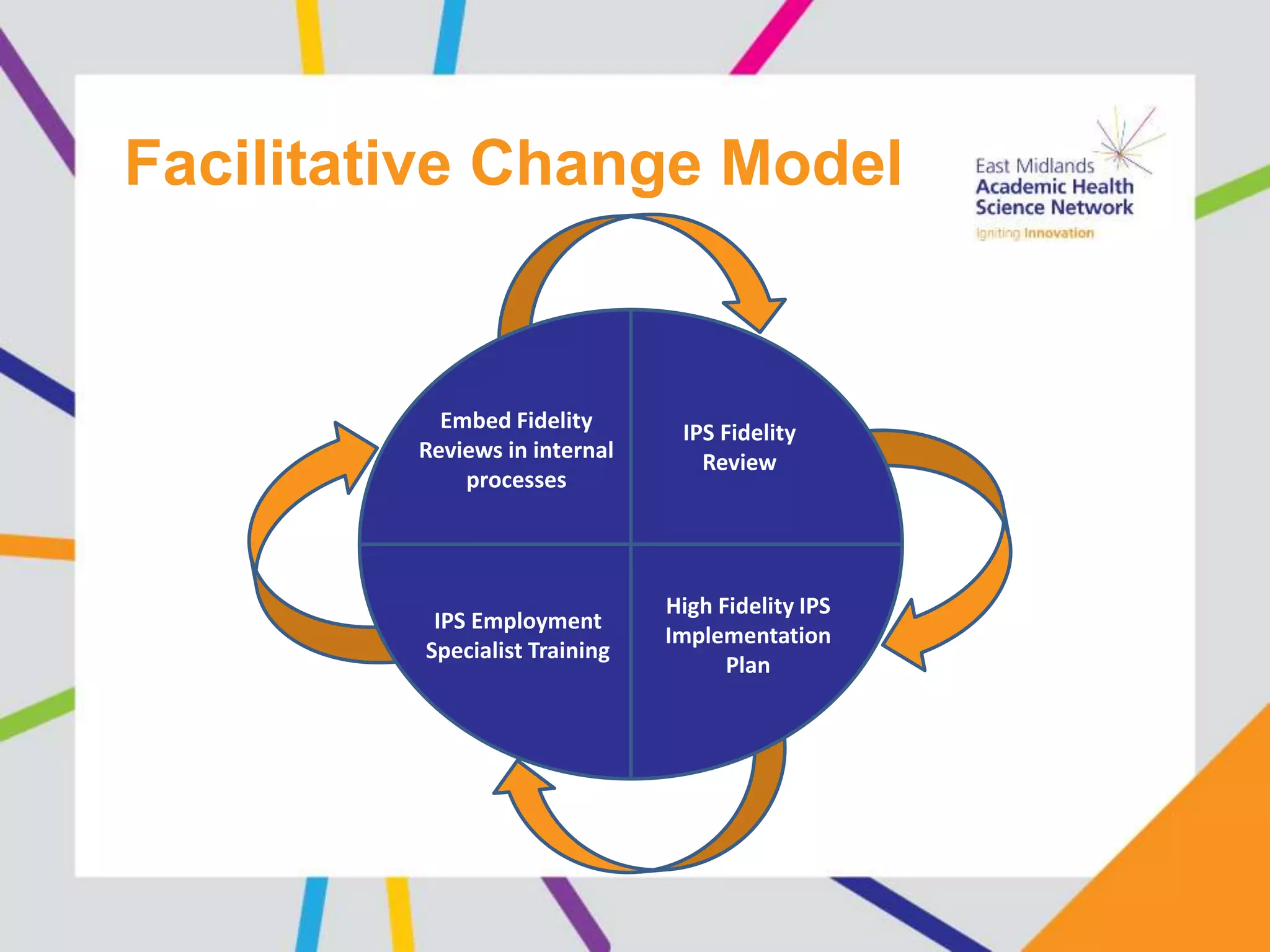
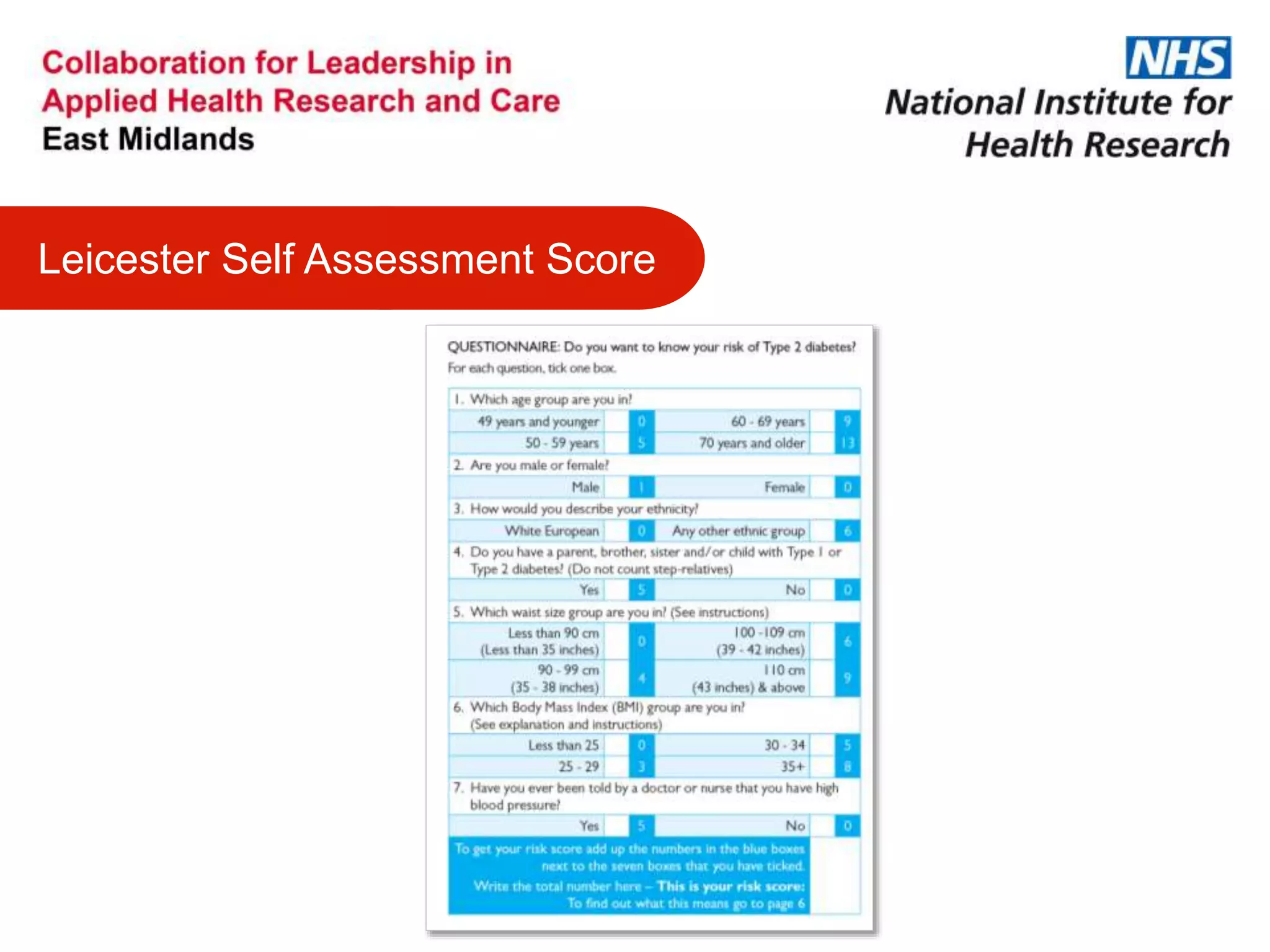
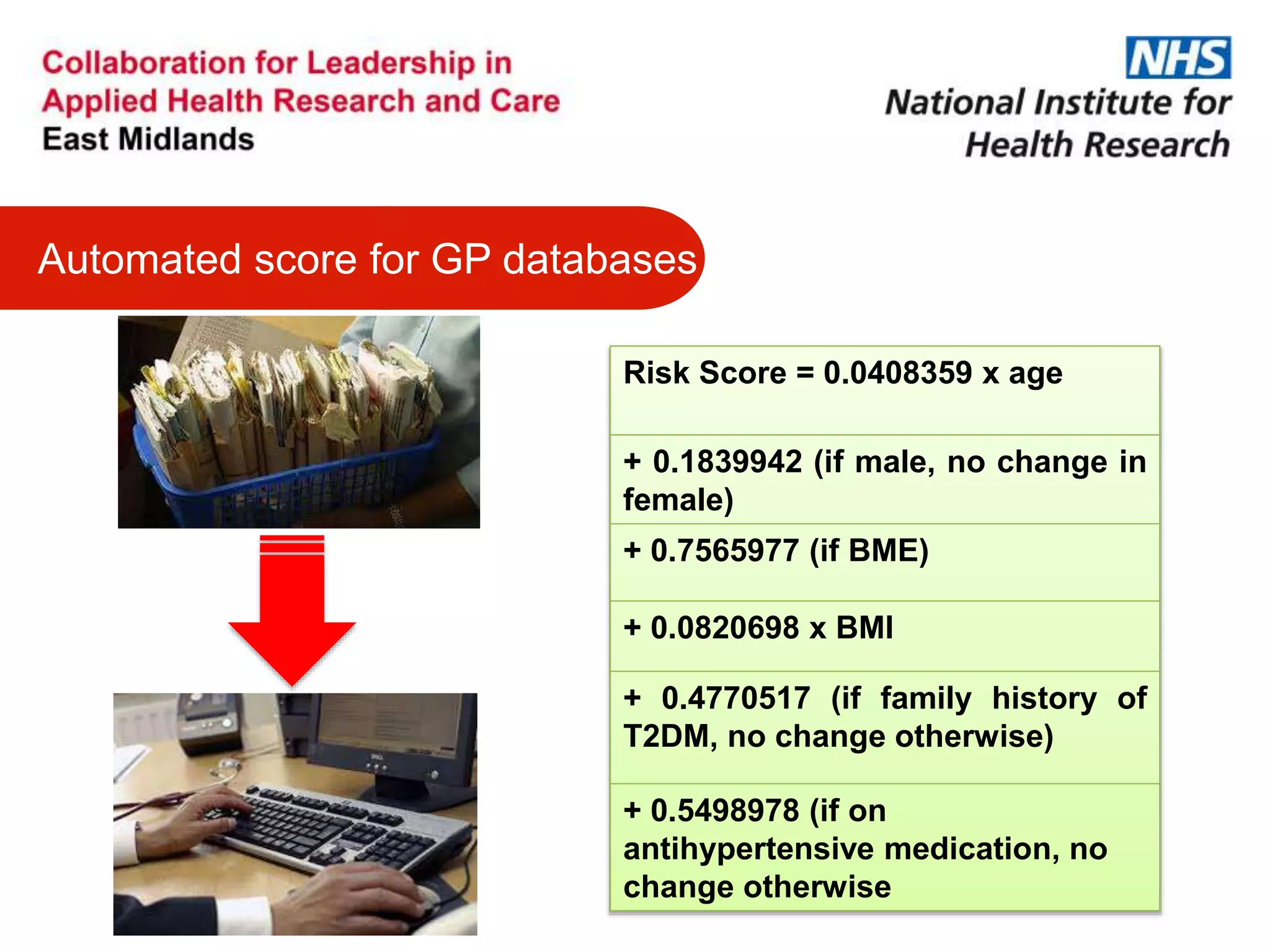
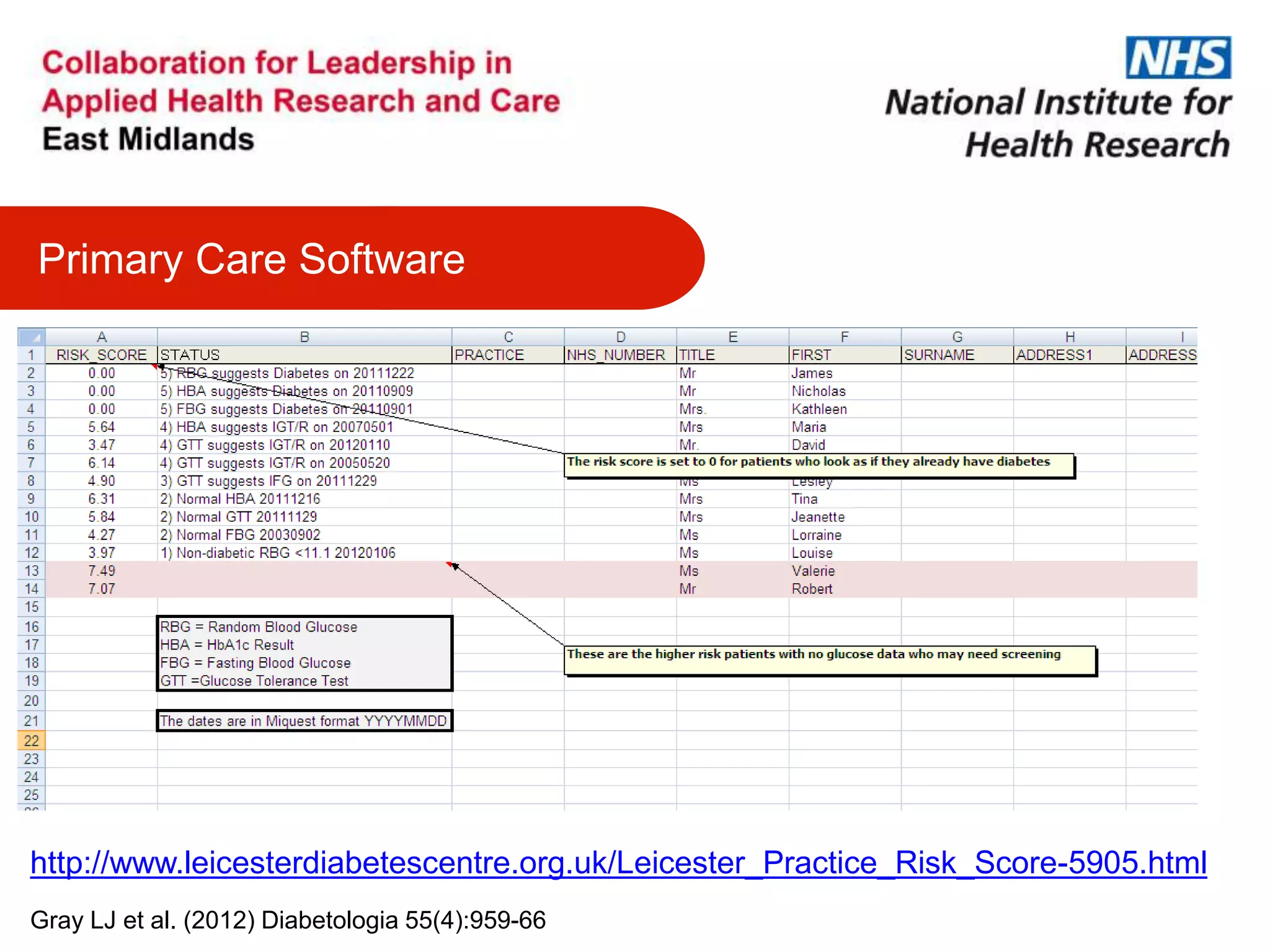
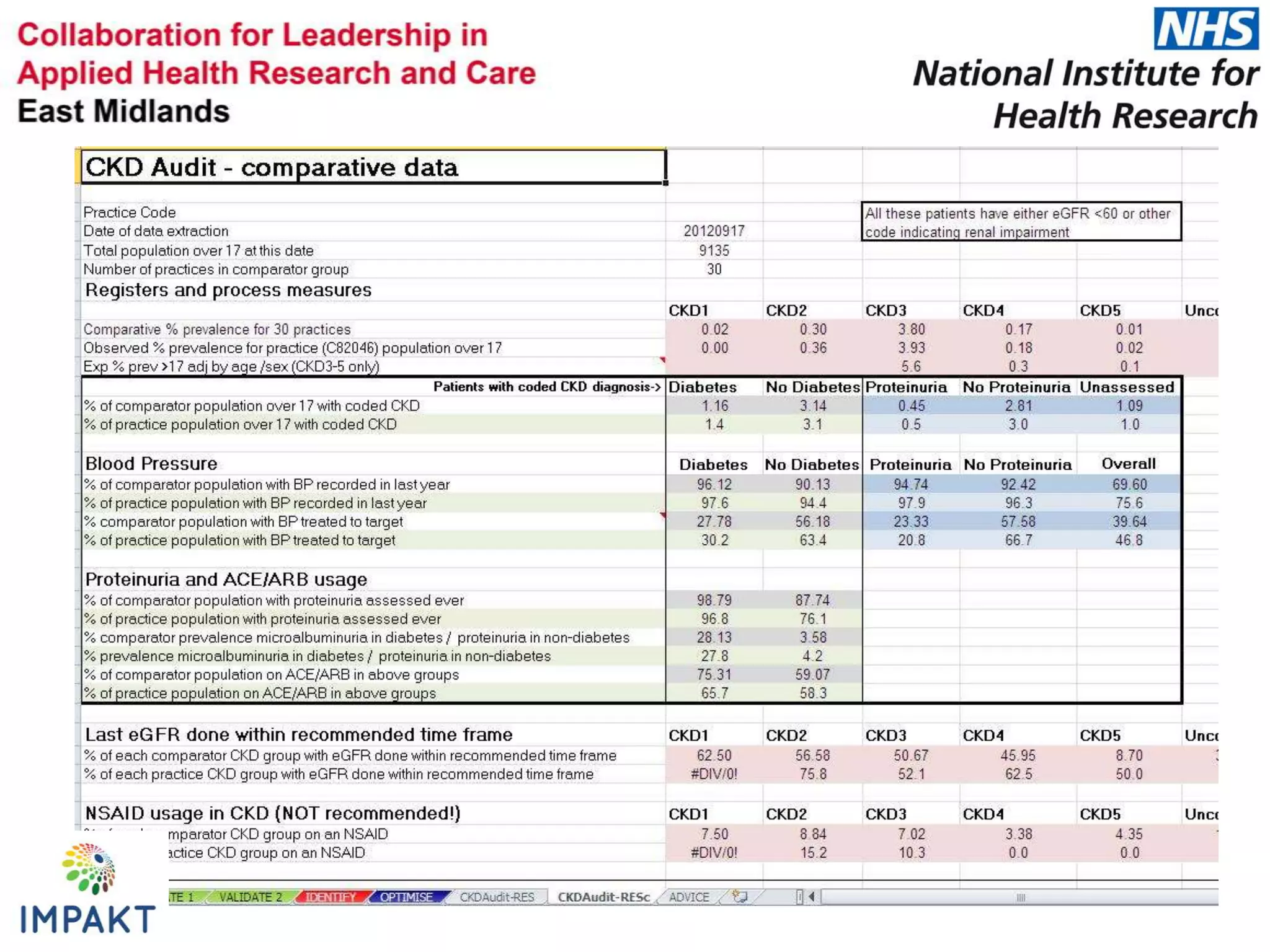
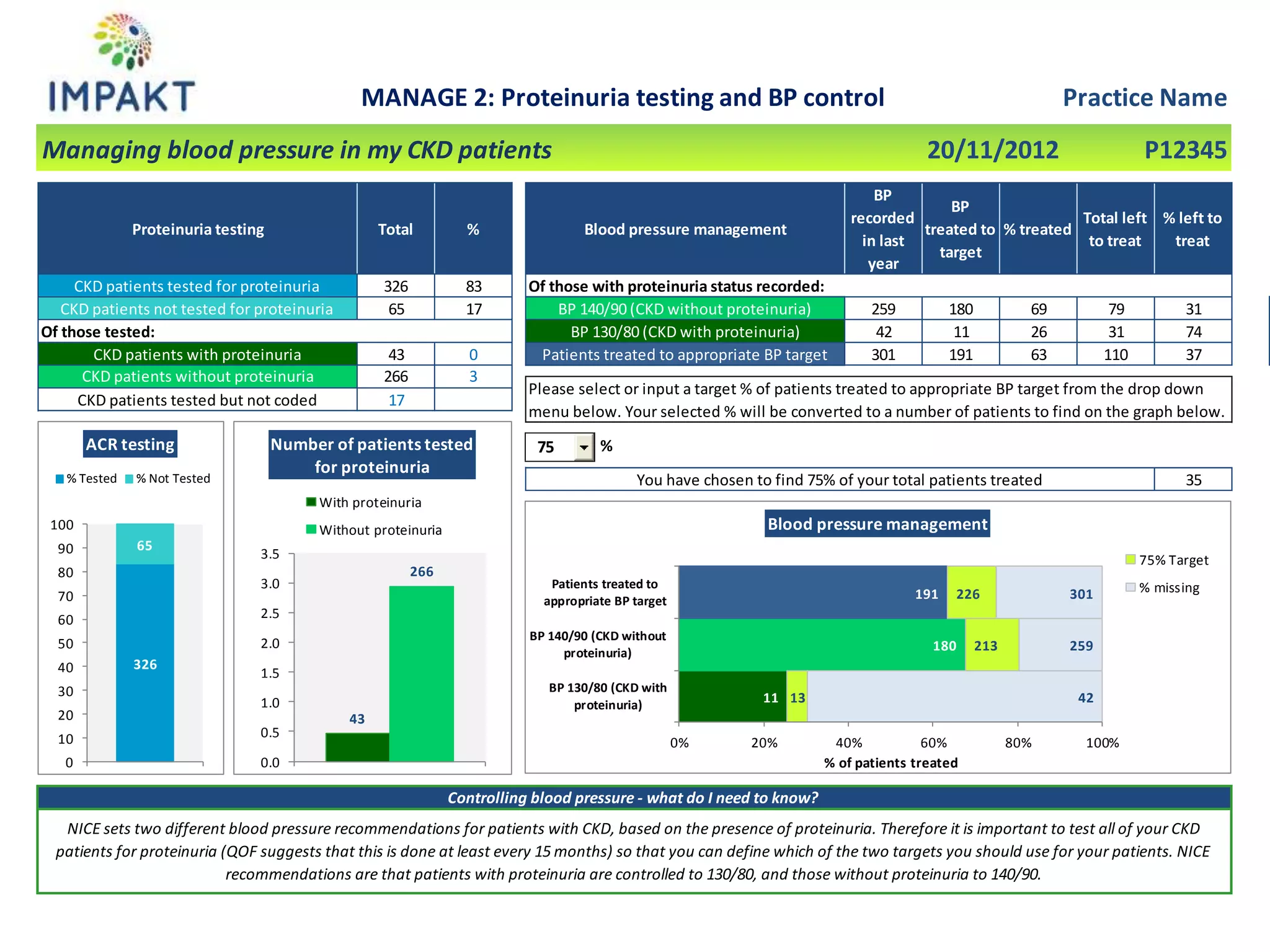
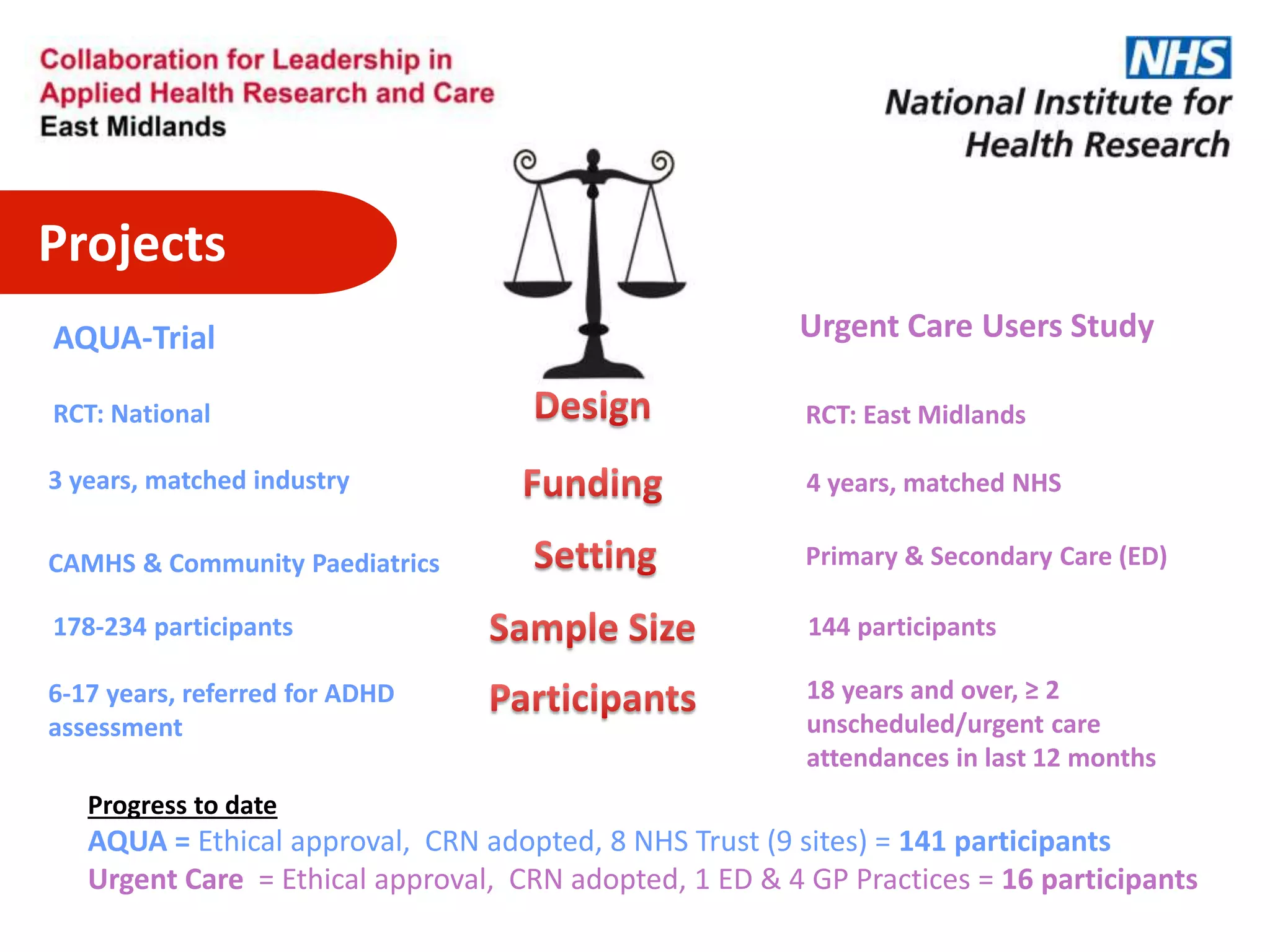
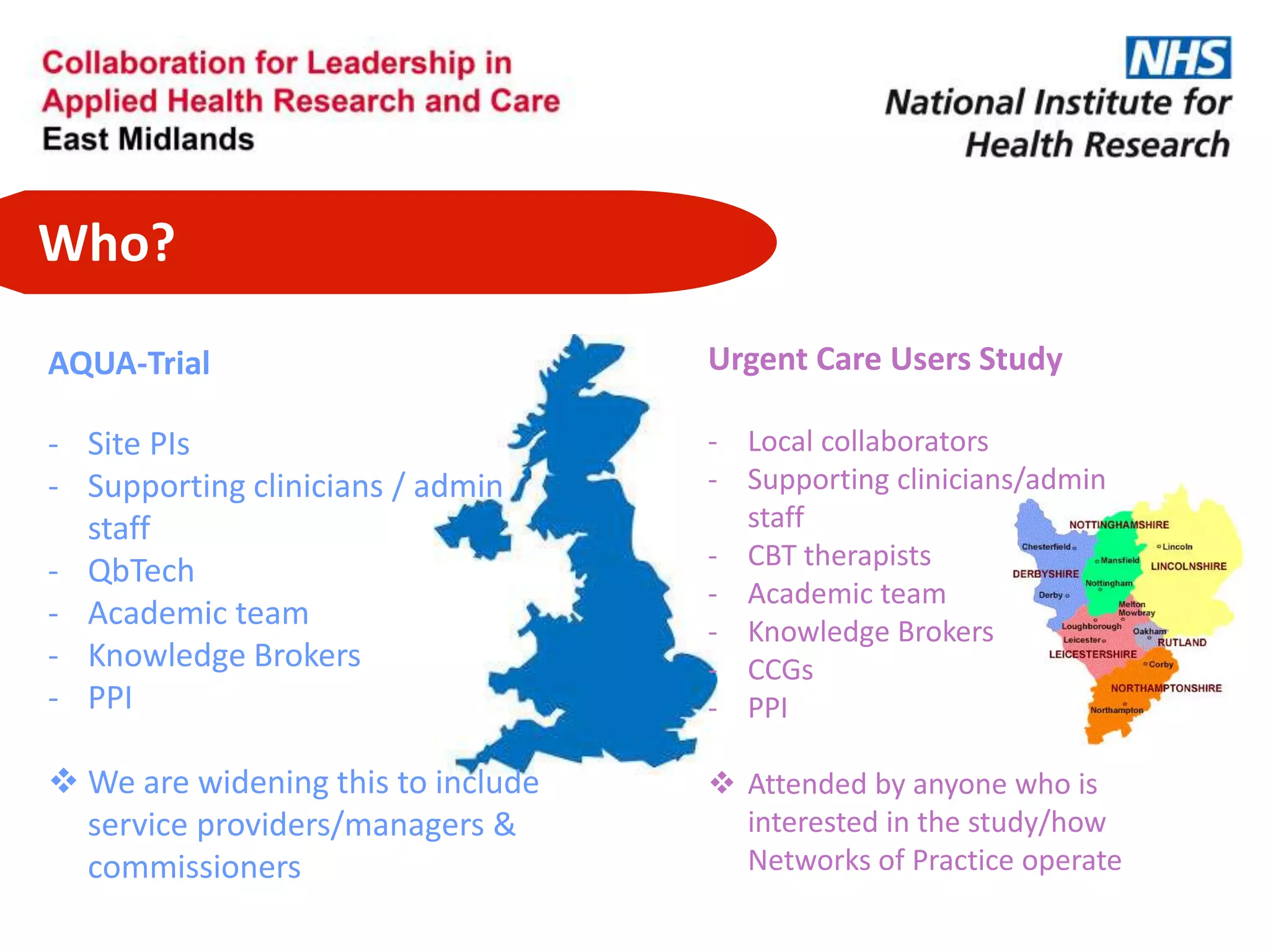
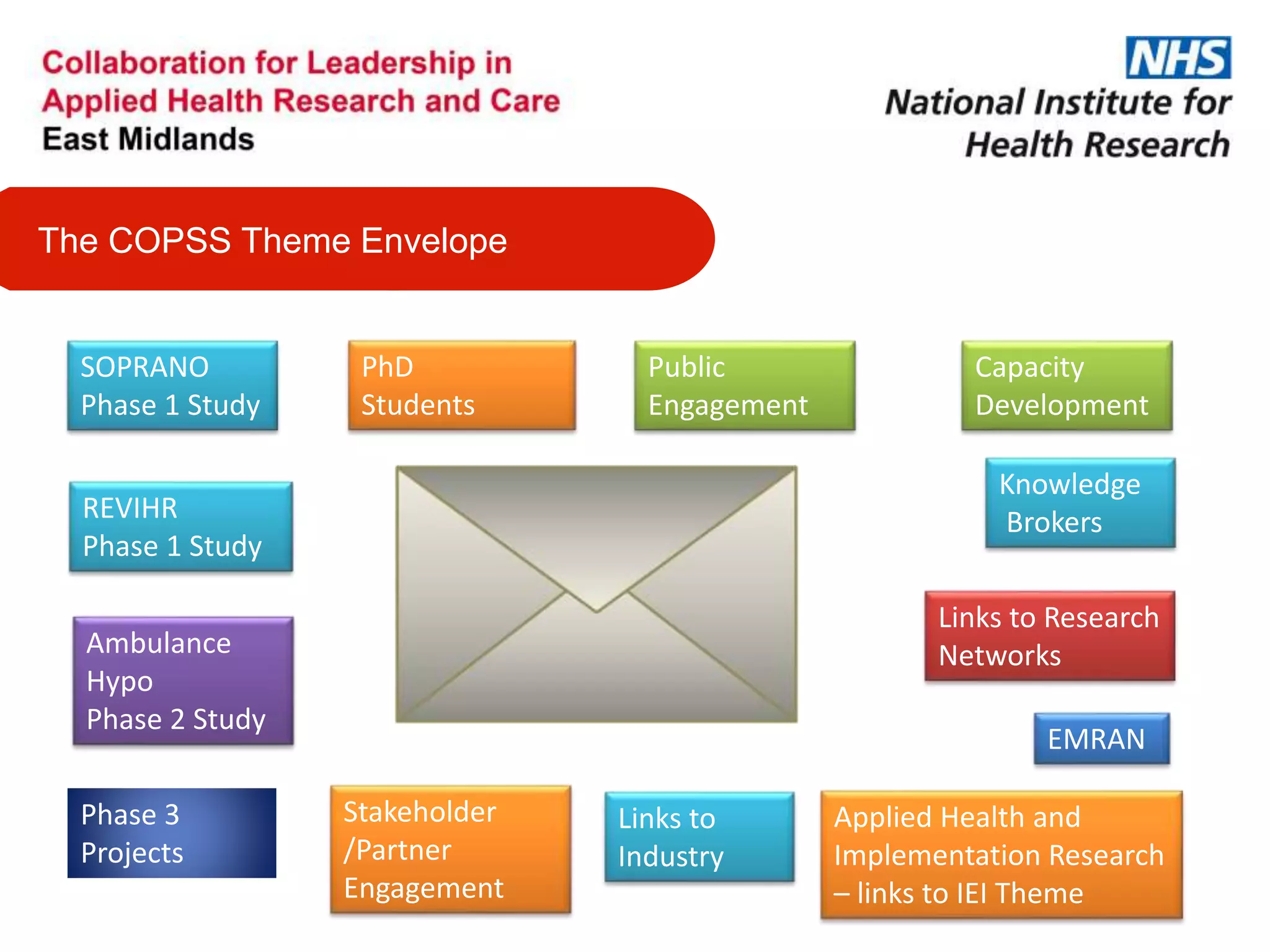
The document provides an overview of the NIHR infrastructure for supporting applied health research in the UK. It discusses how the NIHR was established to improve health outcomes through advancing research, improving NHS care through research participation, strengthening the UK's international research position, and driving economic growth. The NIHR aims to overcome past problems like a lack of research incentives in the NHS, low applied evidence bases, and difficulties developing sustainable research capacity. It created a national health research system to integrate patients, the NHS, universities, investigators and other stakeholders.