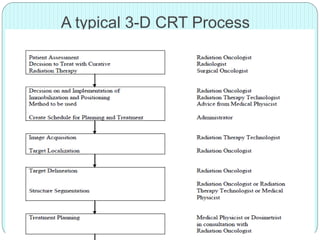
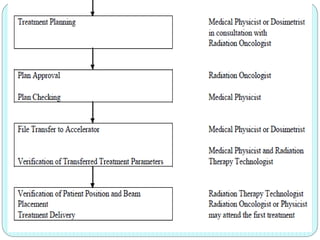
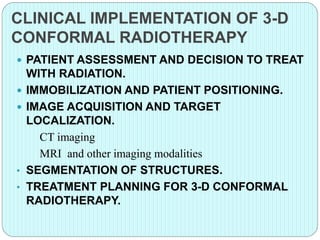
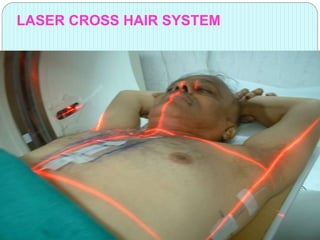
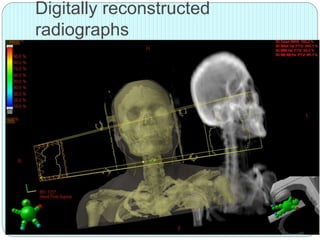
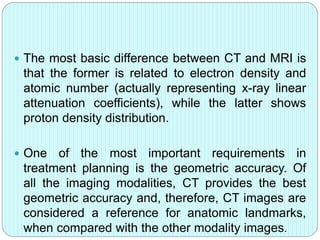
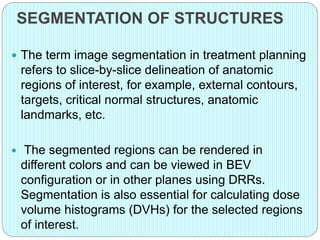
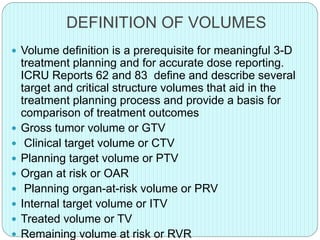
3-D conformal radiation therapy (3-D CRT) aims to conform the high radiation dose region to the target volume while reducing dose to surrounding normal tissues. The process involves immobilizing the patient, obtaining images like CT and MRI to define targets and organs at risk, delineating these structures, and planning radiation beams individually shaped to the target only. Key volumes defined include the gross tumor, clinical target, planning target, and organ at risk volumes.