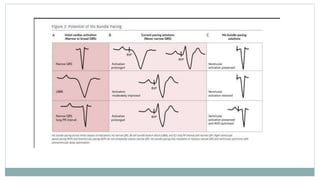
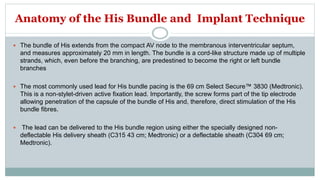
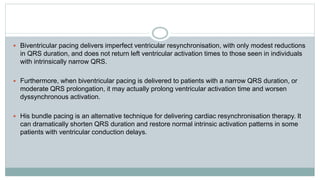
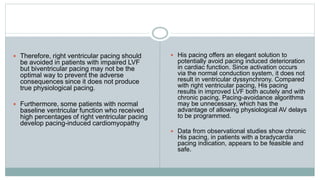
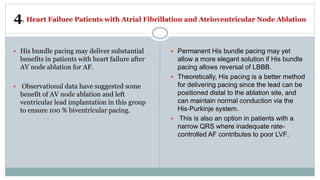
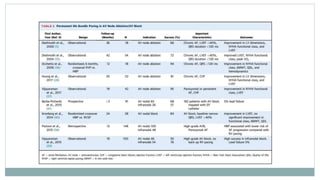
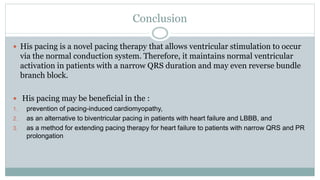
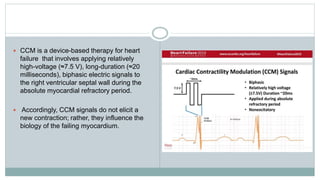
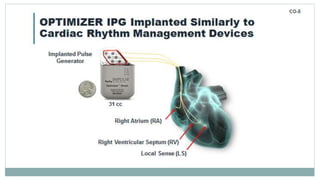
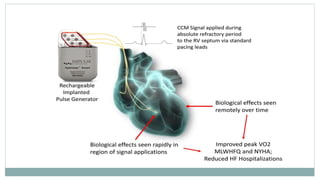
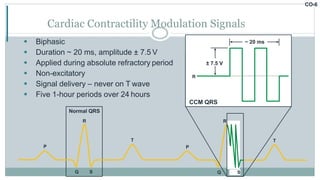
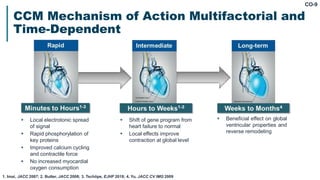
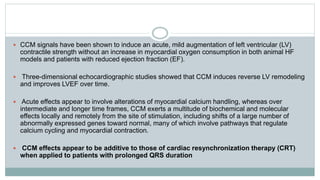
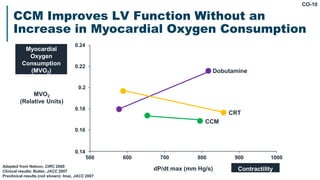
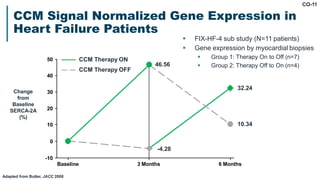
His bundle pacing is an innovative therapy for heart failure that activates the ventricles via the natural conduction system, providing advantages over traditional biventricular pacing, including improved QRS duration and restored intrinsic activation patterns. This technique may benefit patients with left bundle branch block and those with narrow QRS durations while preventing pacing-induced cardiomyopathy. The document also discusses cardiac contractility modulation, a device-based therapy for heart failure that enhances myocardial function without increasing oxygen consumption.











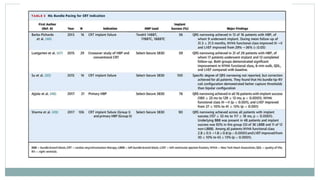
![ Ajijola et al. reported on the first case series of primary HBP (HBP lead in lieu of traditional LV lead) in
CRT-eligible patients . Among 21 patients LBBB, 4 right bundle branch block [RBBB]), permanent HBP was
achieved in 16 patients (76%). The majority of patients demonstrated QRS narrowing with nonselective
capture, with an average QRS reduction of approximately 30%, but not to <120 ms for the majority of
patients.
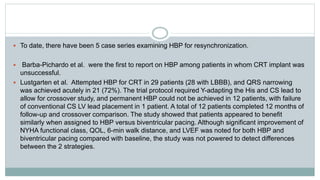
Most recently, Sharma et al. pooled data from 5 centers and compiled the largest retrospective case series
of CRT-eligible patients thus far. They recognized 2 important cohorts:
1. Group I, patients in whom prior CRT had been attempted but was unsuccessful and HBP was used as a bail-out
strategy; and
2. Group II, primary HBP for CRT-eligible patients (AV block, post-AV junction ablation, underlying BBB, or patients
undergoing planned upgrade due to >40% RV pacing). Over a mean follow-up period of 14 months, patients
demonstrated QRS narrowing, improvement in NYHA functional class, and LVEF. Implant success was high (95 of 106
patients, 90%) and lead-related complication rate was overall low (7 of 95 patients, 7.3%). Importantly, BBB was present
in 48 patients (45%), and HBP was effective in this group (92% implant success).](https://image.slidesharecdn.com/newhfmodalitieshbpccm-200912052843/85/New-Heart-Failure-modalities-HIS-Bundle-Pacing-Cardiac-Contractility-Modulation-18-320.jpg)











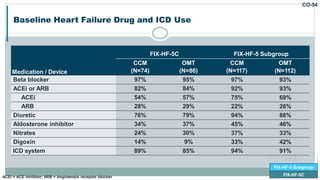
![FIX-HF-5: Clinically Meaningful Improvements in Peak VO2 and QoL
0.24
-0.41
-0.75
-0.50
-0.25
0.00
0.25
0.50
0.75
CCM
(N=179)
OMT
(N=168)
∆
Peak VO2
(mL/kg/min)
[SD]
0.65 mL/kg/min
Nominal p=0.024
-15.4
-5.7
-20
-15
-10
-5
0
CCM
(N=196)
OMT
(N=184)
∆
MLWHFQ
(Total Score)
[SD]
-9.66 Total Score
Nominal p < 0.0001
FIX-HF-5
FIX-HF-5 Subgroup
FIX-HF-5C](https://image.slidesharecdn.com/newhfmodalitieshbpccm-200912052843/85/New-Heart-Failure-modalities-HIS-Bundle-Pacing-Cardiac-Contractility-Modulation-51-320.jpg)




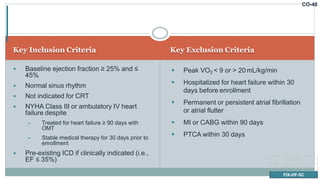
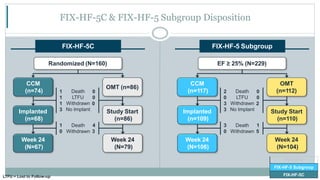
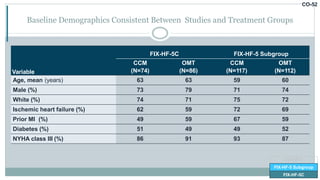
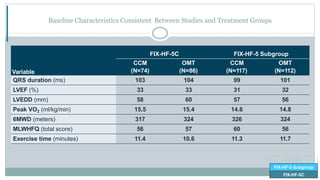


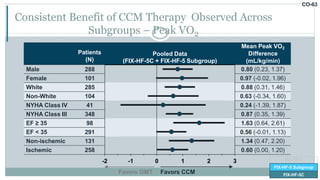
![CO-58
Primary Effectiveness Met Demonstrating Significant Improvement in Peak VO2
Difference (95% CI)
Mean Peak VO2
Difference
(mL/kg/min)
Posterior
Probability
Benefit
Bayesian Model 0.84 (0.12, 1.55) 0.989
-1 0 1 2
Favors OMT Favors CCM
Success = Posterior Probability > 0.975
FIX-HF-5
FIX-HF-5 Subgroup
FIX-HF-5C
ITT Population
FIX-HF-5 Subgroup [CCM=117, OMT=112]
FIX-HF-5C [CCM=74, OMT=86]](https://image.slidesharecdn.com/newhfmodalitieshbpccm-200912052843/85/New-Heart-Failure-modalities-HIS-Bundle-Pacing-Cardiac-Contractility-Modulation-70-320.jpg)
![Peak VO2 in Treatment Effect inCCM Maintained Across 24 Weeks
Peak VO2
(mL/kg/min)
Week
17
16
15
14
13
0 12 24
∆ = 0.675 ∆ = 0.836
OMT
CCM
FIX-HF-5
FIX-HF-5 Subgroup
FIX-HF-5C
ITT Population
FIX-HF-5 Subgroup [CCM=117, OMT=112]
FIX-HF-5C [CCM=74, OMT=86]](https://image.slidesharecdn.com/newhfmodalitieshbpccm-200912052843/85/New-Heart-Failure-modalities-HIS-Bundle-Pacing-Cardiac-Contractility-Modulation-71-320.jpg)


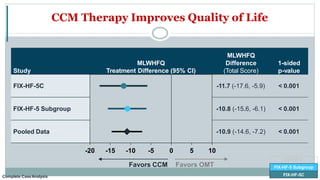
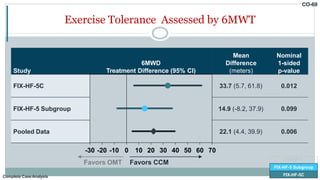
![Change from Baseline to 24 Weeks in 6MWD
FIX-HF-5C
FIX-HF-5
FIX-HF-5 Subgroup
FIX-HF-5C
43.0
9.3
0
20
40
60
80
CCM
(N=69)
OMT
(N=72)
∆
6MWD
(Meters)
[95% CI]
33.7 meters
Nominal p=0.012
Complete CaseAnalysis](https://image.slidesharecdn.com/newhfmodalitieshbpccm-200912052843/85/New-Heart-Failure-modalities-HIS-Bundle-Pacing-Cardiac-Contractility-Modulation-78-320.jpg)

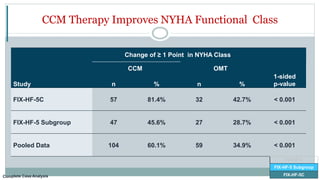
![FIX-HF-5C
CCM
(N=68)
OMT
(N=86) p-value
Freedom from All-Cause Death 98.3% 95.3% 0.255
Freedom from Cardiac Death 100% 96.5% 0.120
Freedom from All-Cause Death or Hospitalization 77.7% 78.1% 0.944
Freedom from CV Death or HF Hospitalization 97.1% 89.2% 0.066
Secondary Safety Endpoints Demonstrated No Significant Safety Concerns
FIX-HF-5
FIX-HF-5 Subgroup
FIX-HF-5C
Per Protocol Population
FIX-HF-5C [CCM=68, OMT=86]](https://image.slidesharecdn.com/newhfmodalitieshbpccm-200912052843/85/New-Heart-Failure-modalities-HIS-Bundle-Pacing-Cardiac-Contractility-Modulation-80-320.jpg)
![CO-79
FIX-HF-5C: 97% CCM Patients Free From Cardiovascular Death or HF Hospitalization
FIX-HF-5C
CCM OMT
Freedom from
Cardiac Death or
HF Hospitalization
97.1% (66/68) 89.2% (75/84)
2-sided logrank p=0.066
Patients
with
Event
(%)
Week 24
50
43
Week 12
66
76
Week 0
CCM 68
OMT 84
20%
15%
OMT
CCM
0%
5%
10%
HR=0.26
FIX-HF-5
FIX-HF-5 Subgroup
FIX-HF-5C
Per Protocol Population
FIX-HF-5C [CCM=68, OMT=86]](https://image.slidesharecdn.com/newhfmodalitieshbpccm-200912052843/85/New-Heart-Failure-modalities-HIS-Bundle-Pacing-Cardiac-Contractility-Modulation-81-320.jpg)