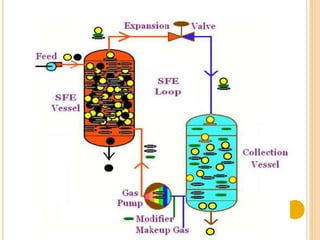
This document discusses equipment and techniques used in near critical fluid (NCF) extraction and its industrial applications. NCF extraction uses fluids near their critical point, like carbon dioxide, which have properties between gases and liquids. Equipment for NCF extraction includes pumps, extraction chambers, recovery chambers, and collection devices. The extraction process involves pressurizing a fluid like CO2, placing a mixture in the extraction chamber, separating components based on solubility, then recovering and collecting the extracted material. NCF extraction offers advantages over organic solvents by being inexpensive, faster, and more environmentally friendly. It has various industrial uses such as extraction, drying, chromatography, and chemical reactions.