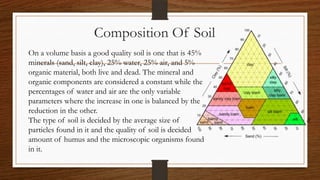
The document discusses the carbon cycle. It begins by explaining that carbon is exchanged among the biosphere, atmosphere, hydrosphere, geosphere, and pedosphere. It then provides details on the global carbon budget, noting that the oceans contain 71% of the world's carbon while terrestrial ecosystems hold around 3%. The atmosphere contains around 1% of total carbon. It concludes by outlining the main components of the carbon cycle as the atmosphere, terrestrial biosphere, oceans, and sediments.























![Global Carbon Budget
The global carbon budget is the balance of the exchanges (incomes
and losses) of carbon between the carbon reservoirs or between one
specific loop (e.g., atmosphere ↔ biosphere) of the carbon cycle. An
examination of the carbon budget of a pool or reservoir can provide
information about whether the pool or reservoir is functioning as a
source or sink for carbon dioxide. The carbon cycle was initially
discovered by Joseph Priestley and Antoine Lavoisier, and popularized
by Humphrey Davy.
The total carbon pool, estimated at about 49,000 metric gigatons (1
metric gigaton equals 109 metric tons), is distributed among organic
and inorganic forms. Fossil carbon accounts for 22 percent of the total
pool. The oceans contain 71 percent of the world's carbon, mostly in the
form of bicarbonate and carbonate ions. An additional 3 percent is in
dead organic matter and phytoplankton. Terrestrial ecosystems, in
which forests are the main reservoir, hold about 3 percent of the total
carbon. The remaining 1 percent is held in the atmosphere, circulated,
and used in photosynthesis.
Carbon pools in the major reservoirs on earth.[2]
Pool Quantity (gigatons)
Atmosphere 720
Oceans (total) 38,400
Total inorganic 37,400
Total organic 1,000
Surface layer 670
Deep layer 36,730
Lithosphere
Sedimentary carbonates > 60,000,000
Kerogens 15,000,000
Terrestrial biosphere (total) 2,000
Living biomass 600 - 1,000
Dead biomass 1,200
Aquatic biosphere 1 - 2
Fossil fuels (total) 4,130
Coal 3,510
Oil 230
Gas 140
Other (peat) 250](https://image.slidesharecdn.com/chemistryprojectnaturalresources-130607034230-phpapp01/85/natural-resources-29-320.jpg)



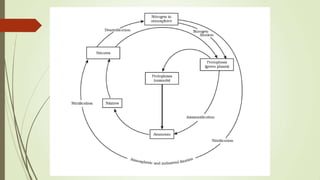
![Nitrogen cycle
The nitrogen cycle is the process by which nitrogen is converted
between its various chemical forms. This transformation can be
carried out through both biological and physical processes.
Important processes in the nitrogen cycle
include fixation, ammonification, nitrification, and denitrification. The
majority of Earth's atmosphere (78%) is nitrogen,[1] making it the
largest pool of nitrogen. However, atmospheric nitrogen has limited
availability for biological use, leading to a scarcity of usable nitrogen
in many types of ecosystems. The nitrogen cycle is of particular
interest to ecologists because nitrogen availability can affect the
rate of key ecosystem processes, including primary
production and decomposition. Human activities such as fossil fuel
combustion, use of artificial nitrogen fertilizers, and release of nitrogen
in wastewater have dramatically altered the global nitrogen cycle.](https://image.slidesharecdn.com/chemistryprojectnaturalresources-130607034230-phpapp01/85/natural-resources-33-320.jpg)