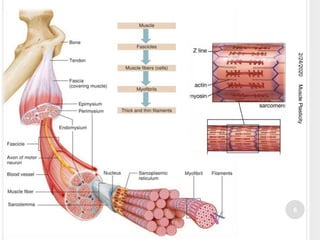
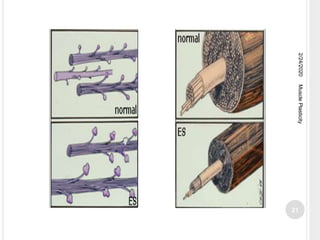
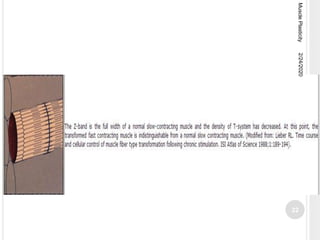
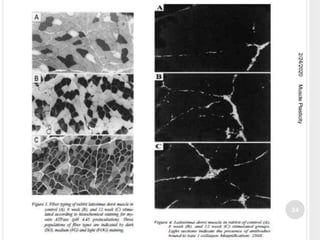
This document discusses muscle plasticity, which refers to the ability of skeletal muscle to adapt structurally and functionally in response to environmental changes such as increased or decreased activity levels. It provides definitions and history of the concept. It describes the effects of chronic low frequency electrical stimulation on muscles, including fiber type transformations, increased mitochondria and vascularization, and changes to contractile properties. Over time periods of hours to weeks, stimulated muscles demonstrate metabolic and structural adaptations that increase their fatigue resistance and transform them from fast-twitch to slow-twitch phenotypes. Several studies are summarized that investigate muscle adaptations to long-term stimulation in animals and humans.