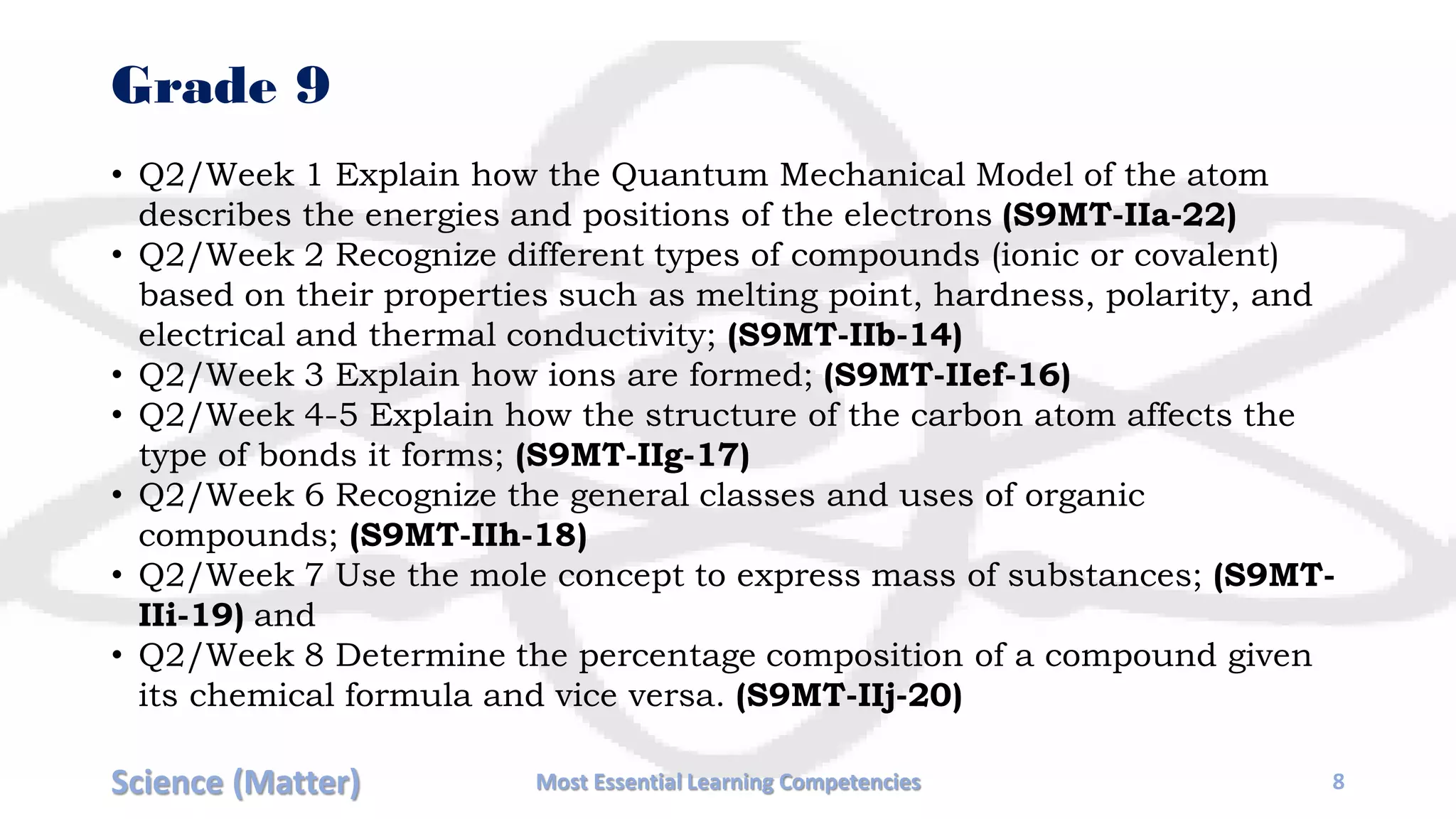
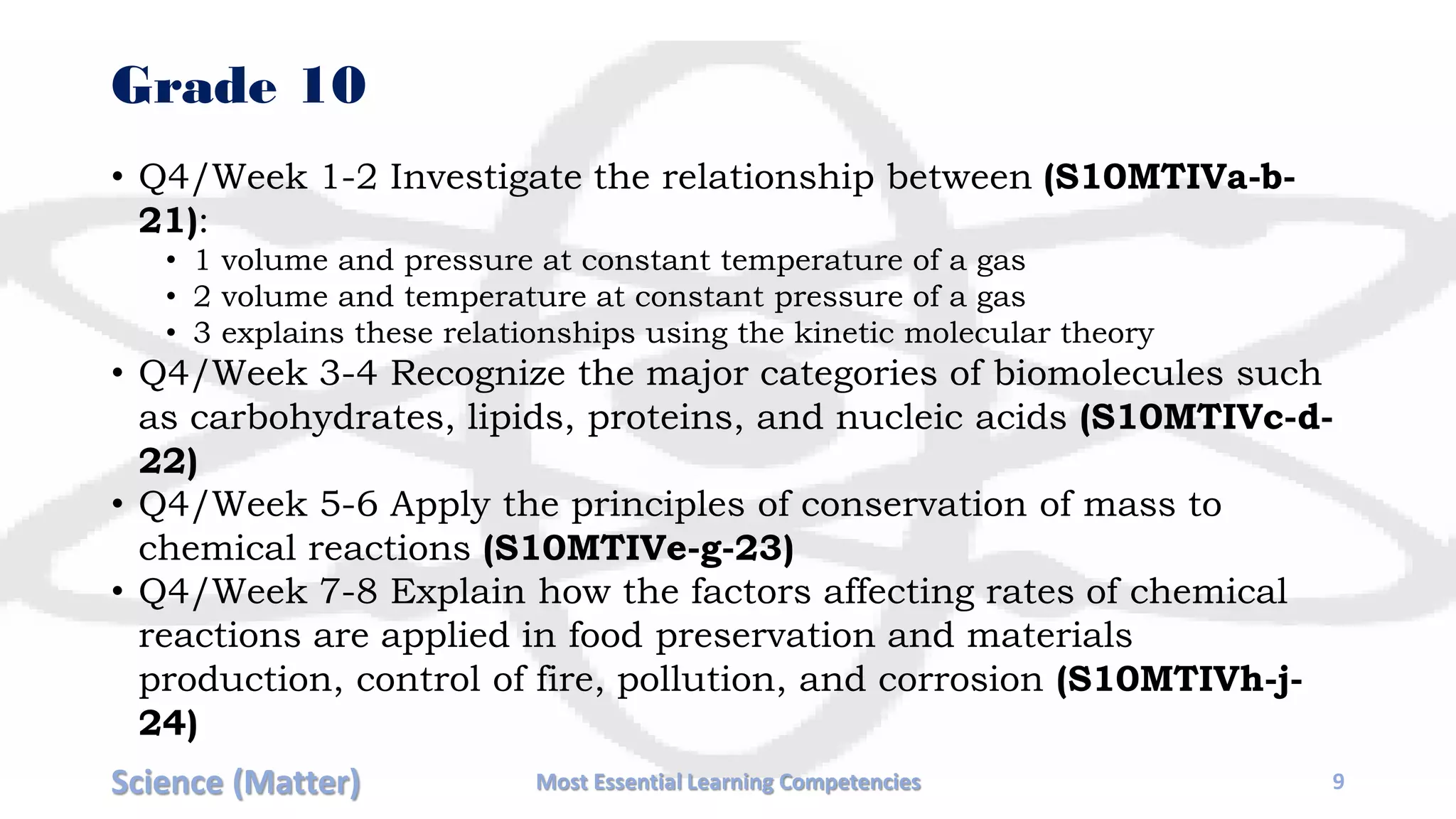
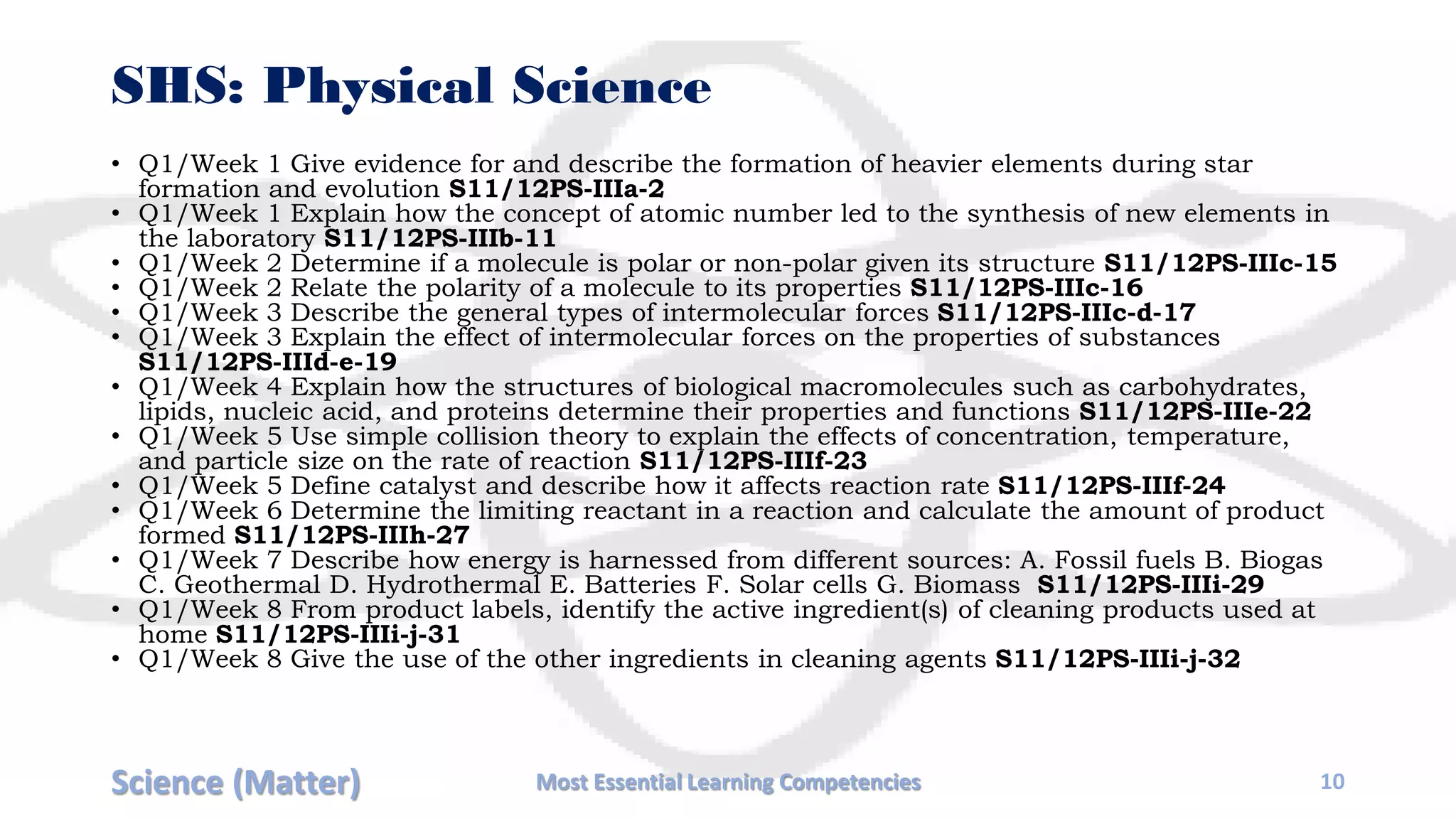
The document outlines the essential learning competencies for science focusing on the topic of matter across various grade levels, from Grade 3 to Senior High School (SHS). It details specific learning goals, such as classifying materials, describing changes in states, and investigating properties of substances and solutions. Each grade level's objectives are organized by week, covering fundamental topics that progress in complexity, aligning with educational standards.