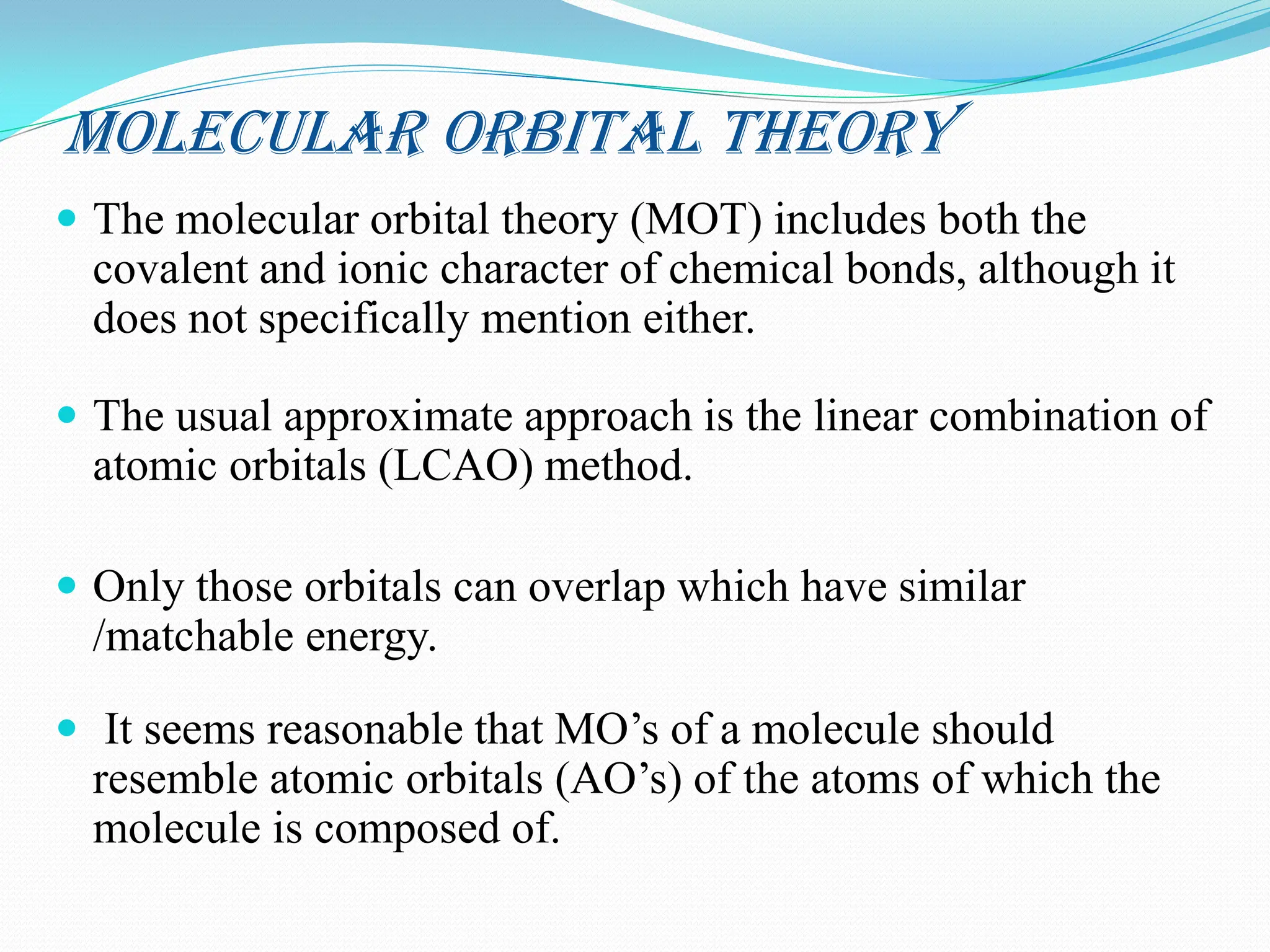
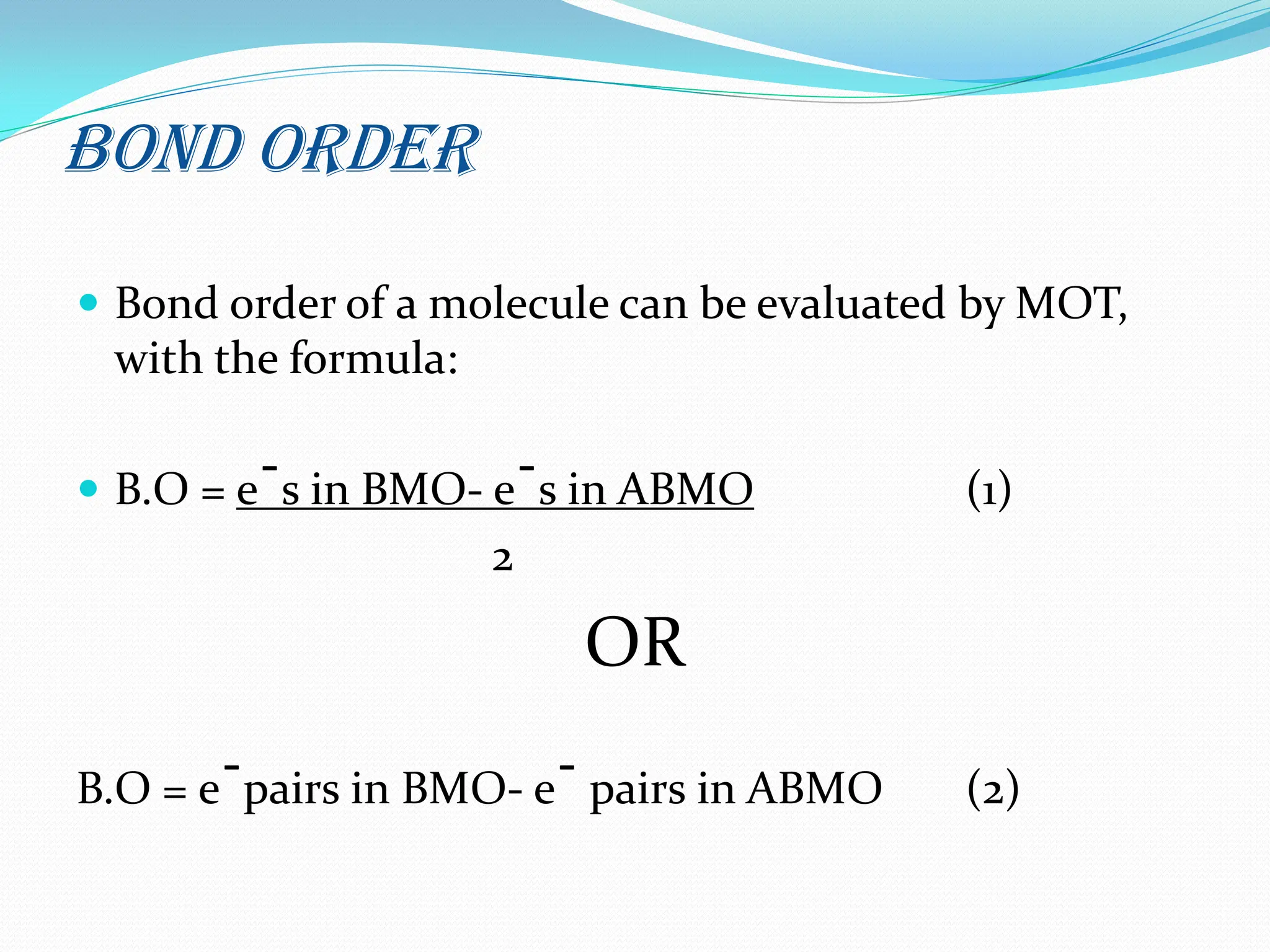
The document discusses the limitations of crystal field theory (CFT), highlighting its inability to address various phenomena such as ligand interactions and the behavior of certain metal-ligand combinations. It contrasts CFT with molecular orbital theory (MOT), which incorporates both covalent and ionic bonding characteristics, emphasizing the significance of molecular orbitals and their energy levels in bond formation. Additionally, the document includes assignments related to molecular orbital diagrams and properties of specific molecules.












![MO Diagram of
high spin [cof6]3-
and
low spin [Co(nh3)6]3+
Molecules](https://image.slidesharecdn.com/lec10-mot-250113095113-b692582e/75/molecular-orbital-thoery-and-MOT-diagrams-explain-in-detail-15-2048.jpg)
![[cof6]3-
Complex
high spin
d6 system
parmagnetic Co(III)
[CoF6]
3-
6F
-](https://image.slidesharecdn.com/lec10-mot-250113095113-b692582e/75/molecular-orbital-thoery-and-MOT-diagrams-explain-in-detail-16-2048.jpg)
![[Co(nh3)6]3+
Complex
d6 system
low spin
dimagnetic
Co(III)
[Co(NH3)6]
3+
6NH3](https://image.slidesharecdn.com/lec10-mot-250113095113-b692582e/75/molecular-orbital-thoery-and-MOT-diagrams-explain-in-detail-17-2048.jpg)
![MO Diagram of high spin [cof6]3-
and low spin [Co(nh3)6]3+
Molecules](https://image.slidesharecdn.com/lec10-mot-250113095113-b692582e/75/molecular-orbital-thoery-and-MOT-diagrams-explain-in-detail-18-2048.jpg)

