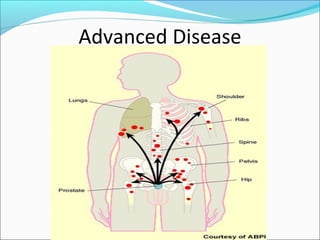
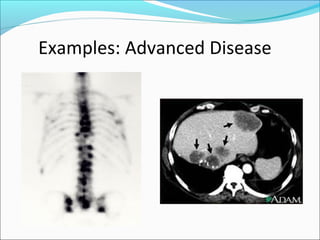
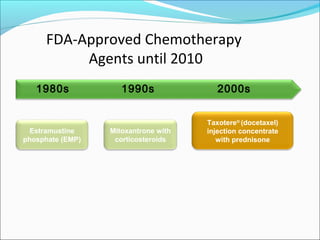
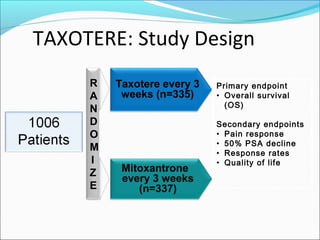
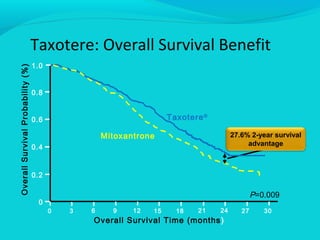
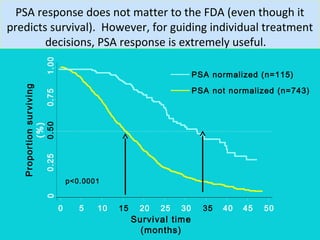
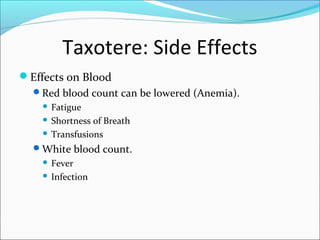
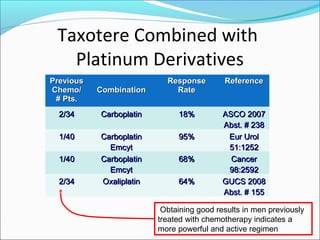
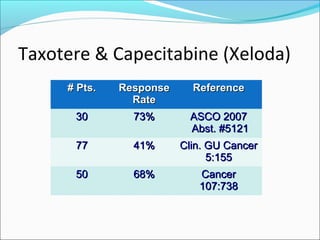
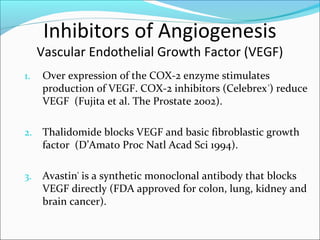
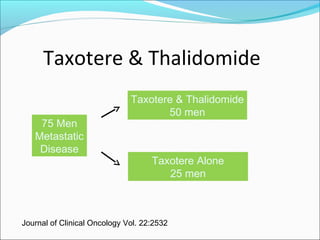
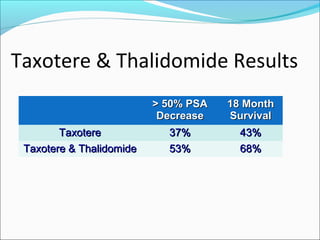
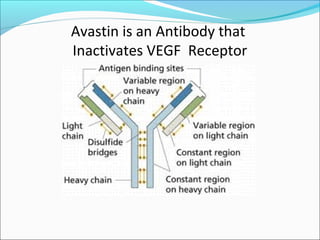
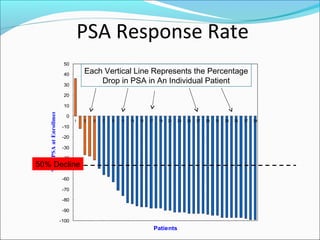
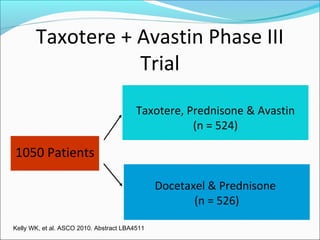
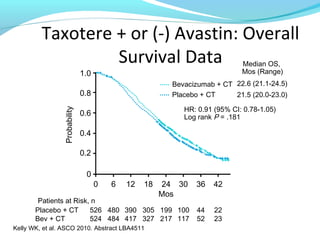
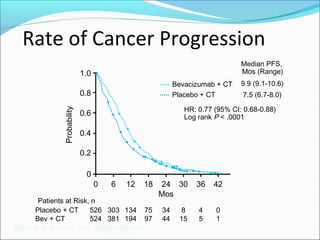
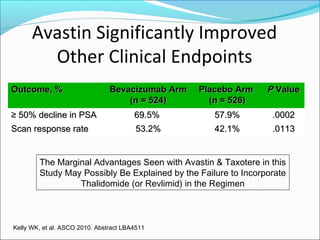
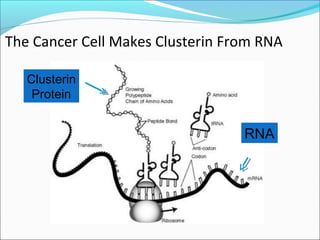
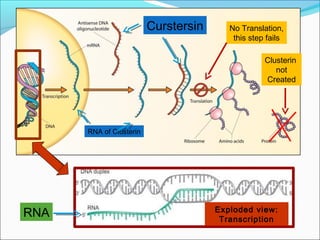
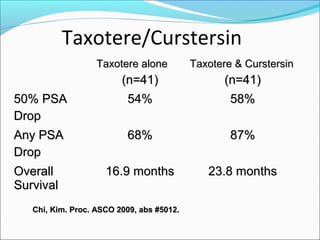
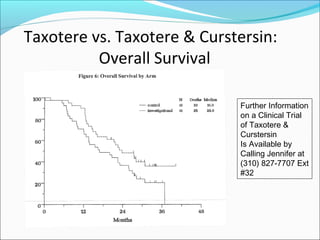
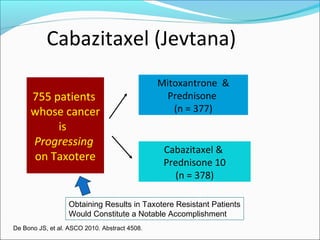
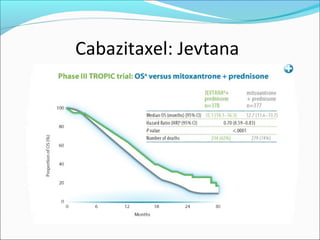
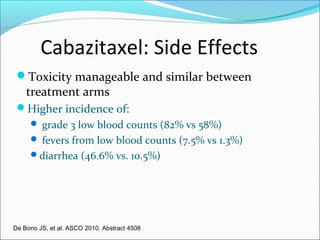
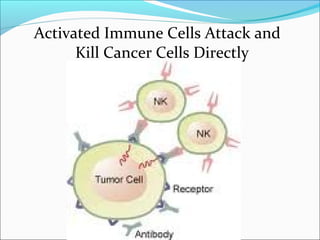
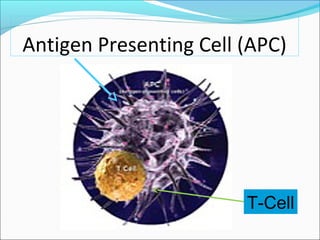
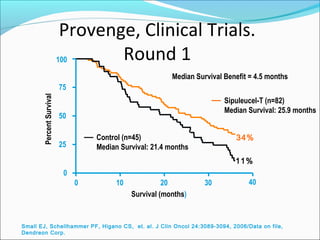
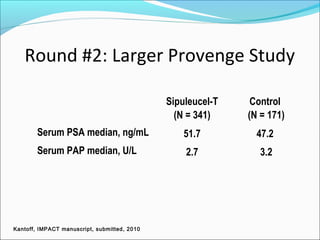
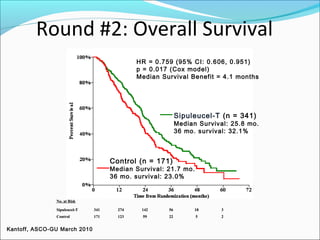
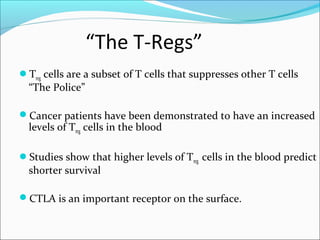
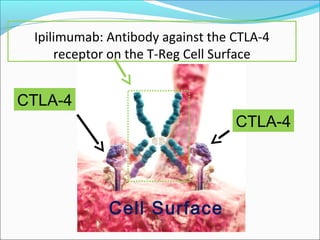
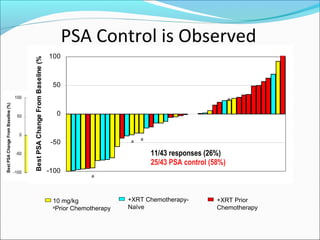
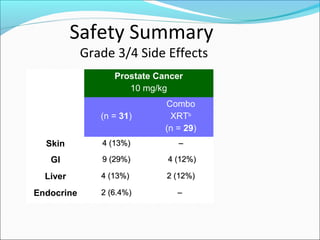
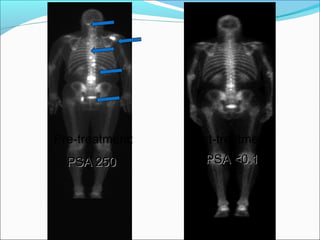
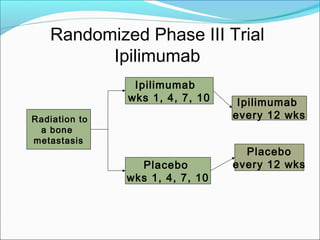
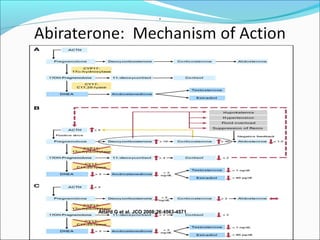
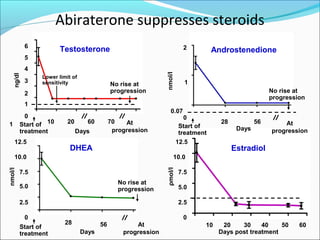
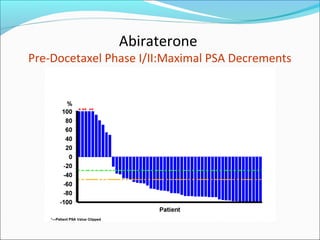
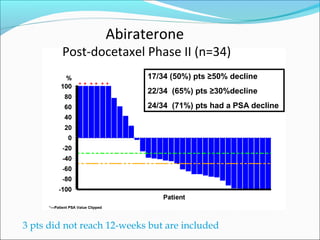
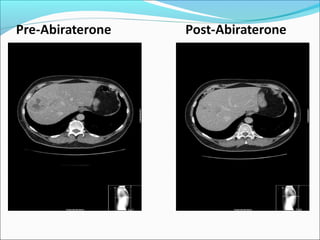
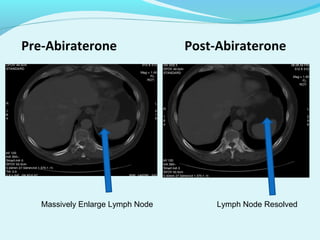
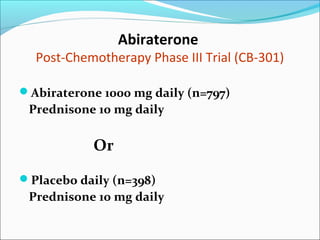
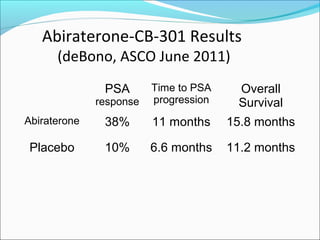
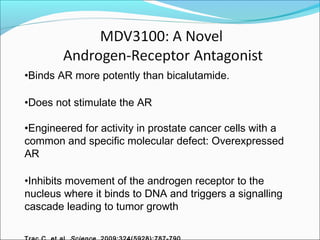
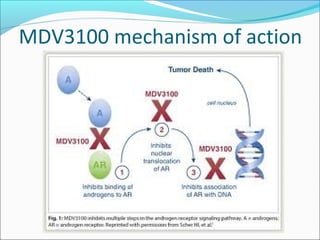
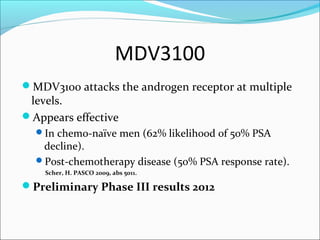
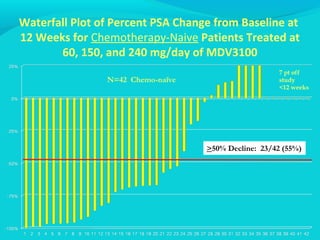
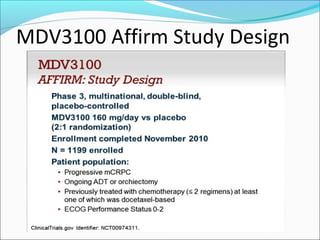
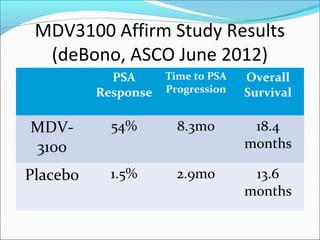
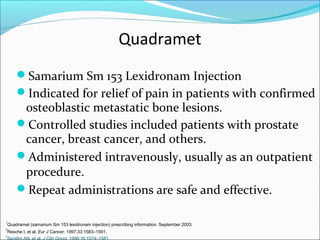
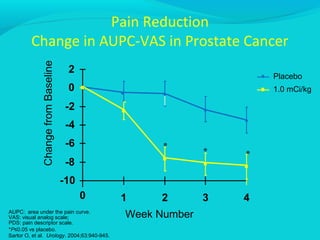
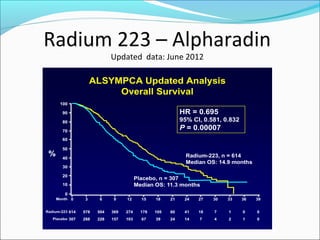
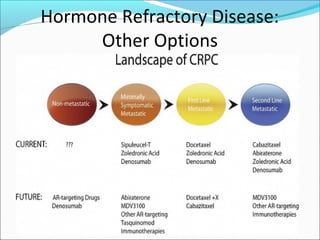
This document discusses treatments for advanced prostate cancer. It begins by describing androgen deprivation therapy as the first line treatment. It then discusses newer FDA-approved treatments since 2010 like cabazitaxel, abiraterone, and immunotherapy drug Provenge. The rest of the document focuses on chemotherapy drug Taxotere/docetaxel, providing details on its efficacy compared to other drugs in clinical trials, side effects, and promising combinations with other treatments like Avastin, carboplatin, and thalidomide. It also discusses other emerging treatments like cabazitaxel, curcustersin, and immunotherapy drug Provenge.