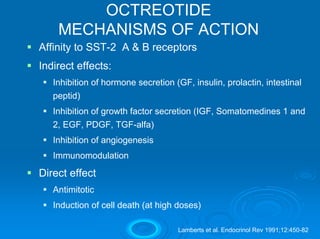
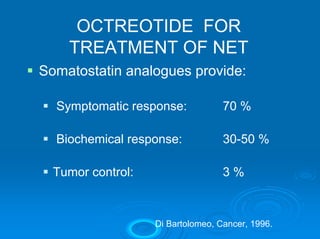
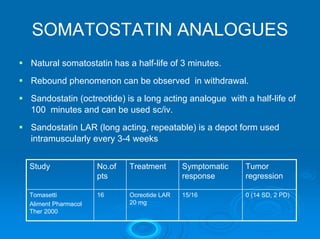
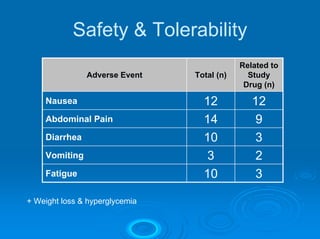
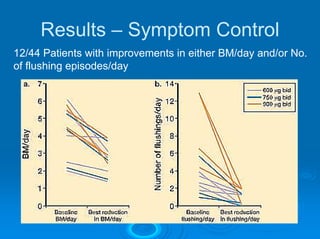
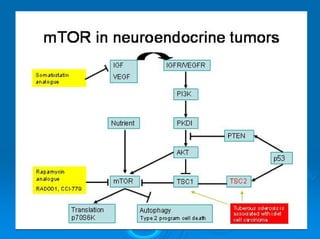
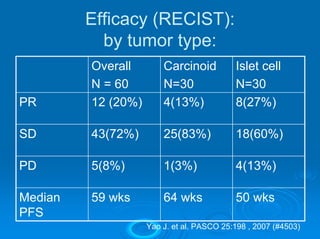
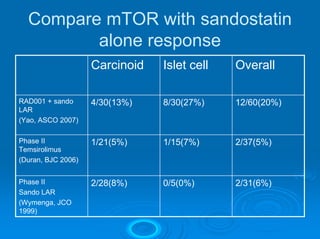
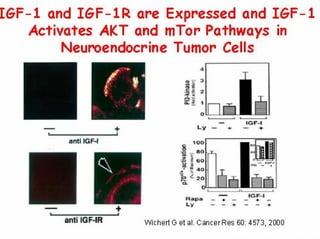
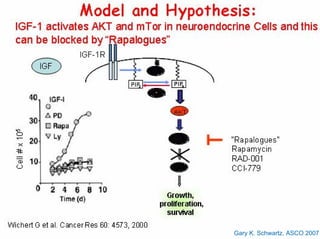
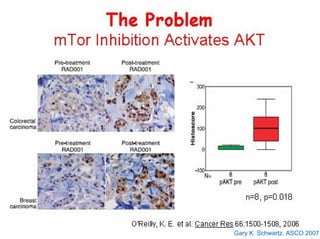
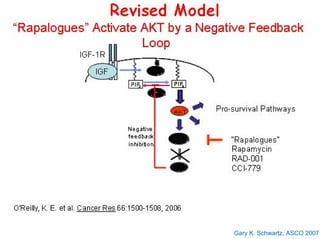
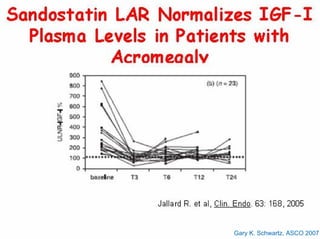
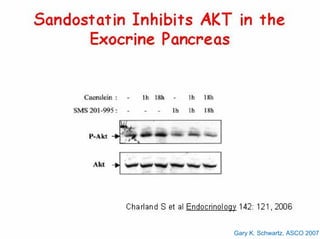
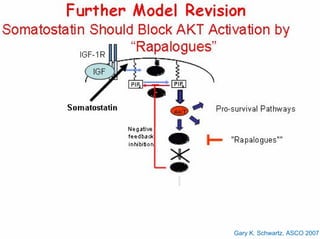
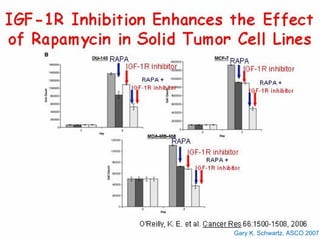
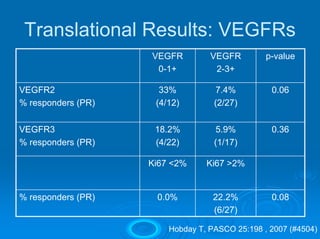
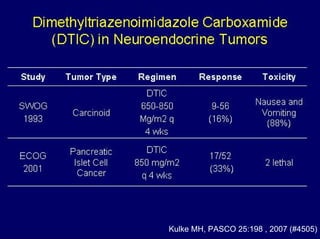
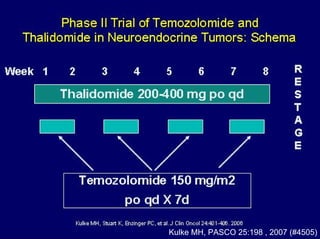
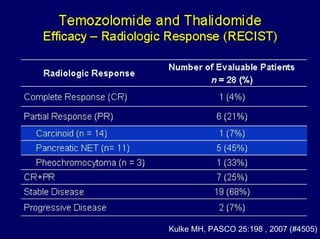
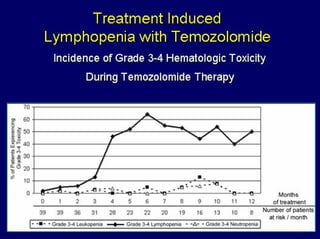
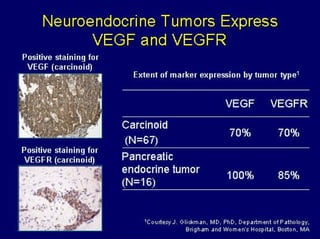
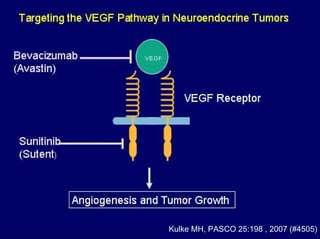
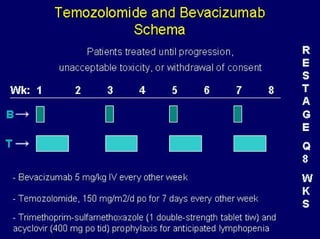
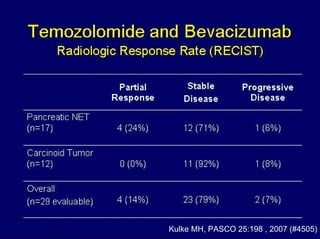
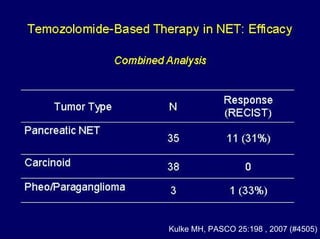
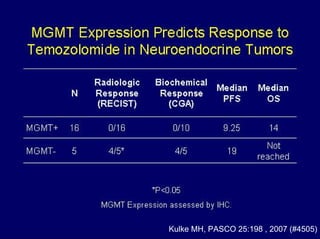
This document summarizes recent advances in the treatment of neuroendocrine tumors. Several new agents have shown promising results in phase II trials, including pasireotide for tumors resistant to octreotide, everolimus combined with octreotide, sorafenib, sunitinib, and temsirolimus. These agents have multiple mechanisms of action and have led to partial responses and prolonged progression-free survival in early studies. While significant progress has been made, treatment of neuroendocrine tumors remains a work in progress as several phase III trials are currently evaluating mTOR and tyrosine kinase inhibitors.