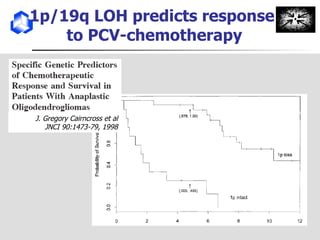
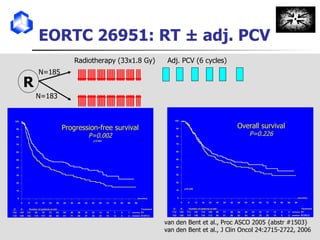
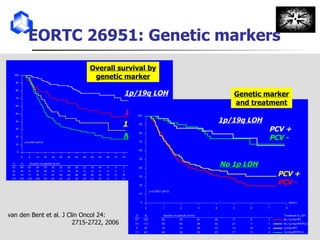
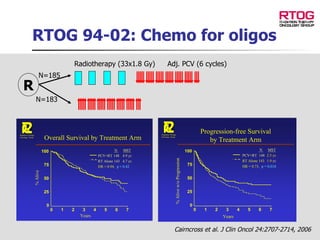
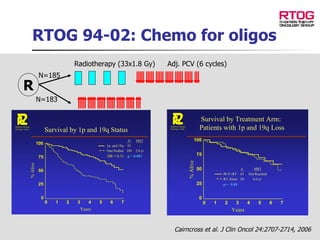
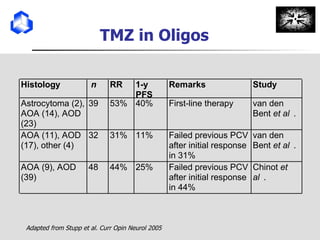
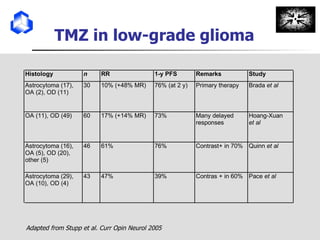
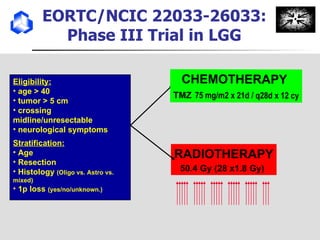
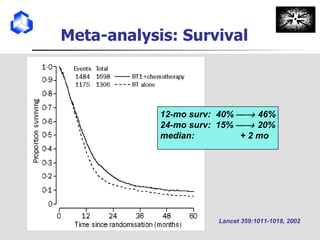
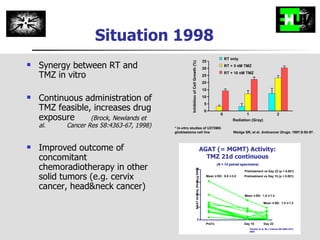
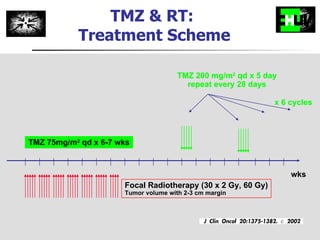
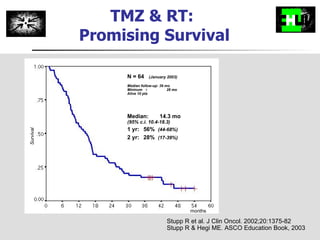
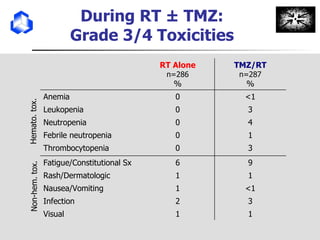
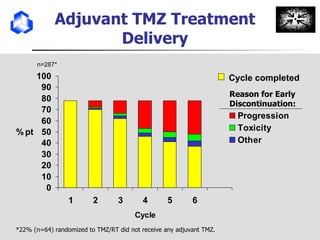
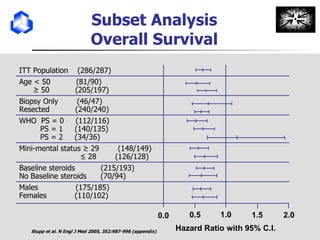
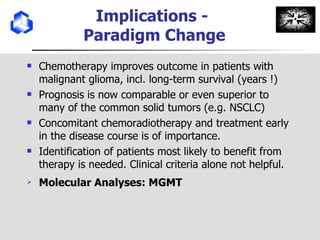
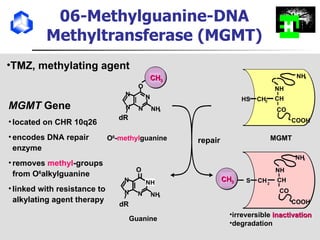
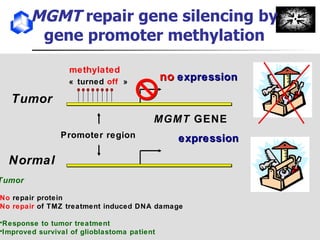
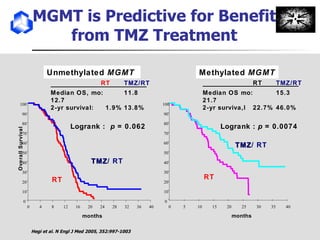
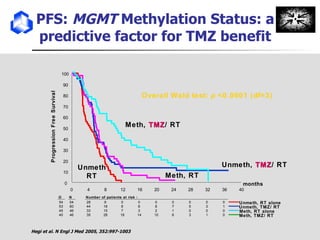
The document discusses optimizing chemotherapy for malignant glioma. Meta-analyses showed that combining temozolomide (TMZ) with radiotherapy improved survival rates compared to radiotherapy alone. A phase III trial demonstrated that concomitant and adjuvant TMZ with radiotherapy significantly improved progression-free and overall survival. Subset analyses found the benefit was consistent across patient subgroups. Methylation of the MGMT gene promoter was identified as predictive of benefit from TMZ therapy.


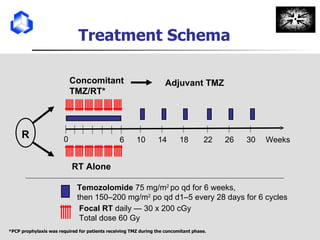
![Progression Free Survival months 0 6 12 18 24 30 36 42 0 10 20 30 40 50 60 70 80 90 100 RT TMZ/RT Median PFS, mo: 5.0 6.9 1-yr PFS: 9% 27% 2-yr PFS: 2% 11% HR [95% C.I.]: 0.54 [0.45-0.64] p <0.0001 TMZ/RT RT TMZ/RT RT % Stupp et al. N Engl J Med 2005, 352:987-996 O N Number of patients at risk : 281 286 104 26 11 4 0 0 260 287 154 77 51 24 8 1](https://image.slidesharecdn.com/optimizingchemotherapyformalignantglioma-100202123142-phpapp01/85/Optimizing-Chemotherapy-For-Malignant-Glioma-13-320.jpg)
![Overall Survival months 0 6 12 18 24 30 36 42 0 10 20 30 40 50 60 70 80 90 100 RT TMZ/RT Median OS, mo: 12.1 14.6 2-yr survival: 10% 26% HR [95% C.I.]: 0.63 [0.52-0.75] p <0.0001 RT TMZ/RT TMZ/RT RT % Stupp et al. N Engl J Med 2005, 352:987-996 O N Number of patients at risk : 261 286 240 144 59 23 2 0 219 287 246 174 109 57 27 4](https://image.slidesharecdn.com/optimizingchemotherapyformalignantglioma-100202123142-phpapp01/85/Optimizing-Chemotherapy-For-Malignant-Glioma-14-320.jpg)
![MGMT Promoter Methylation is Prognostic 0 5 10 15 20 25 30 35 40 0 10 20 30 40 50 60 70 80 90 100 months Overall Survival Unmethylated N = 114 (55%) Methylated, N = 92 (45%) N=206 Unmeth Meth Median OS, mo: 12.2 18.2 HR [95% CI]: 0.45 [0.32-0.61] Logrank test: p <0.0001 Risk of death reduced by 55% Hegi et al. N Engl J Med, 352: 997-1003, 2005](https://image.slidesharecdn.com/optimizingchemotherapyformalignantglioma-100202123142-phpapp01/85/Optimizing-Chemotherapy-For-Malignant-Glioma-19-320.jpg)
![RTOG0525/EORTC Intergroup Phase III Study TMZ daily x 6 wks R Radiotherapy (30 x 2 Gy) Concomitant Phase Adjuvant (maintenance) Phase (6 mo) Dose dense TMZ (100 mg/m2 daily x 21d) Stratify by: MGMT methylation Tissue For additional information contact: RTOG: www.rtog.org or EORTC: www.eortc.be ; or the study chairs: Mark Gilbert: [email_address] or Roger Stupp: [email_address]](https://image.slidesharecdn.com/optimizingchemotherapyformalignantglioma-100202123142-phpapp01/85/Optimizing-Chemotherapy-For-Malignant-Glioma-23-320.jpg)