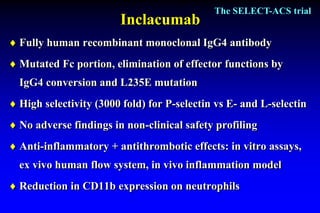
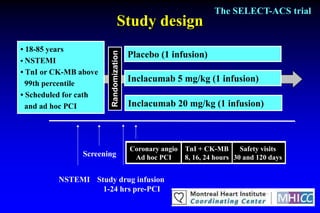
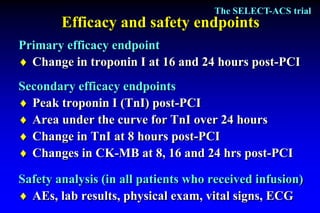
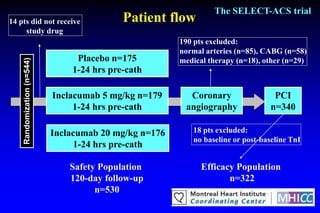
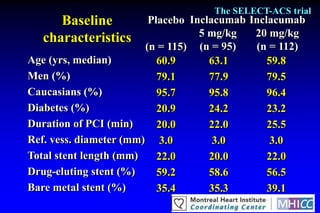
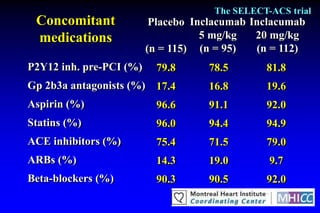
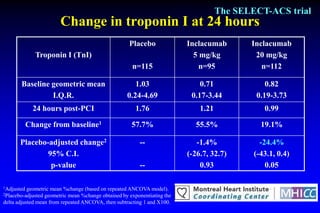
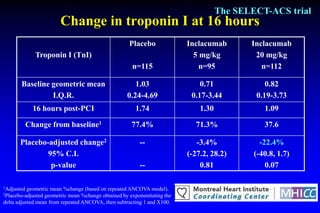
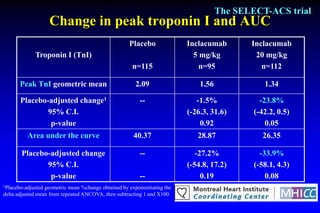
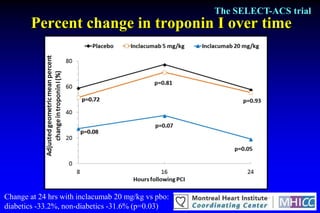
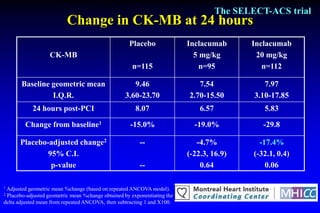
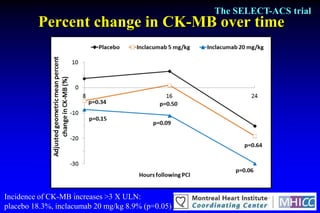
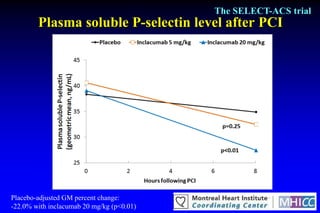
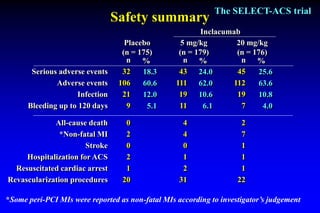
The SELECT-ACS trial evaluated the effects of the p-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention (PCI) in non-ST elevation myocardial infarction (NSTEMI) patients. Results indicate that inclacumab significantly reduced troponin I levels 24 hours post-PCI compared to placebo, suggesting a protective effect against myocardial damage. Further research is necessary to assess the clinical implications of inclacumab in this population.