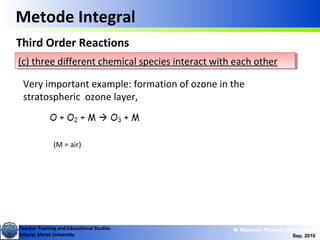
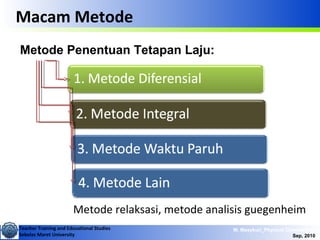
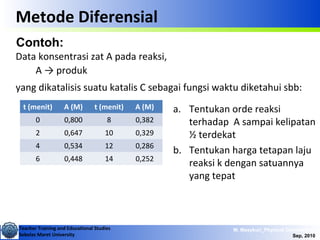
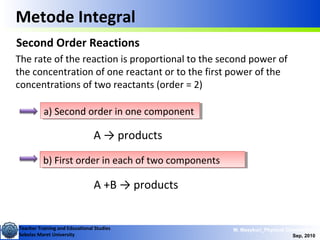
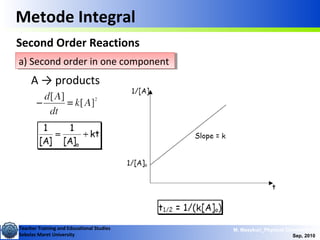
This document discusses methods for determining reaction rates and orders in physical chemistry. It describes differential and integral methods, including graphs of concentration versus time used to determine rate coefficients and reaction orders. Specific reaction orders are examined, such as zero order, first order, and second order reactions. Examples of each type are provided. Third order reactions involving interactions of two and three molecules are also discussed.

![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
Rate of Reaction
Consider a chemical reaction having the overall
stoichiometry:
aA + bB → cC + dD
The Rate of Reaction is defined as
Experimentally we find that
k = rate coefficient
Ci = Concentration of Reactant “i” or [i]
γi = Order of reaction with respect to reactant “i”](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-2-320.jpg)
![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
Metode Diferensial
• Data dikumpulkan sebagai laju perubahan
konsentrasi/waktu terhadap konsentrasi reaktan.
• Contoh dasar persamaan bagi reaksi dengan 2 reaktan:
aA + bB → produk
qp
BAk
dt
Ad
r ][][
][
=−=
]ln[]ln[ln
][
lnln BqApk
dt
Ad
r ++=
−=
• Dibuat kurva ln r terhadap ln [A] atau ln [B]](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-4-320.jpg)
![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
Metode Diferensial
]ln[]ln[ln
][
lnln BqApk
dt
Ad
r ++=
−=
• Kurva ln r terhadap ln [A]
]ln['lnln Apkr +=
Orde reaksi terhadap A = p, diperoleh dari slope = tg α
Tetapan laju k, diperoleh dari intersep grafik
Orde reaksi terhadap A = p, diperoleh dari slope = tg α
Tetapan laju k, diperoleh dari intersep grafik
Slope = tg α
= p
]ln[ln'ln BqkkIntersep +==](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-5-320.jpg)

![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
Metode Integral
• The rate of the reaction is independent of the
concentration of the reacting substance
Zero Order Reactions
A → products
∫∫ −=
−=
=−
dtkAd
k
dt
Ad
k
dt
Ad
A
A
][
][ 0
][
][
][
[A]t - [A]0 = - kt
The reaction half-time t1/2 is the time
required for the concentration to
decrease to one-half of its initial value.](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-8-320.jpg)
![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
Metode Integral
Zero Order Reactions
Grafik [A] versus t,
Persamaan grafik,
Slope = - k
Intersep = [A]0
Waktu paruh,](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-9-320.jpg)
![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
Metode Integral
First Order Reactions
The rate of the reaction is proportional to the first power of the
concentration of the reacting substance (order = 1)
A → products
∫∫ −=
−=
=−
dtk
A
Ad
kdt
A
Ad
Ak
dt
Ad
A
A
][
][ 0
][
][
][
][
][
][
Examples:
• decay of radioactive nuclei fluorescence
• decay of electronically excited molecules
• isomerization of cyclobutene to butadiene
• milk turns sour when left out overnight](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-10-320.jpg)
![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
Metode Integral
First Order Reactions
Grafik ln [A] versus t,
Persamaan grafik,
Buktikan bahwa,](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-11-320.jpg)
![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
b) First order in each of two componentsb) First order in each of two components
Metode Integral
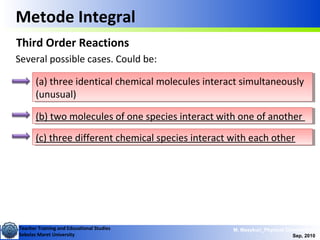
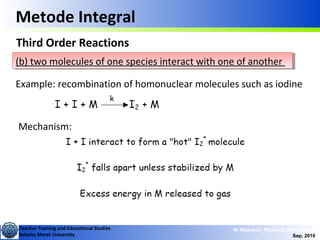
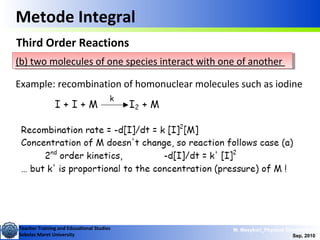
Second Order Reactions
A +B → products
Special cases:
i) [A]o = [B]o
ii) [B]o <<[A]o](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-14-320.jpg)
![Teacher Training and Educational Studies
Sebelas Maret University
M. Masykuri_Physical Chemistry IV
Sep, 2010
b) First order in each of two componentsb) First order in each of two components
Metode Integral
Second Order Reactions
Special cases (cont.):
i) [A]o = [B]o ii) [B]o <<[A]o
This is like 2nd order in
one component
Where,
This is called a pseudo
1st order reaction](https://image.slidesharecdn.com/1modul2persamaanlajureaksi-101114220231-phpapp02/85/modul-persamaan-laju-reaksi-15-320.jpg)