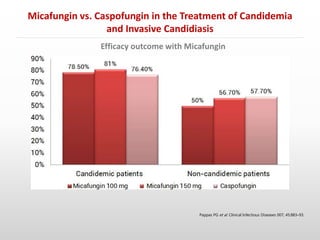
- Micafungin was found to be non-inferior to caspofungin in treating candidemia and invasive candidiasis based on a randomized controlled trial of 595 patients (Pappas et al. 2007). Treatment success rates were similar between micafungin and caspofungin groups.
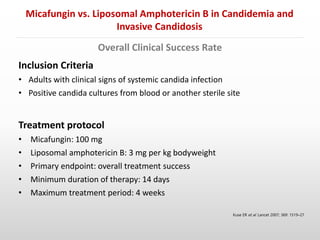
- Another randomized controlled trial by Kuse et al. (2007) found micafungin to be non-inferior to liposomal amphotericin B in treating candidemia and invasive candidiasis based on intention-to-treat analysis of 537 patients, with similar treatment success rates of 89.6% for micafungin and 89.5% for liposomal