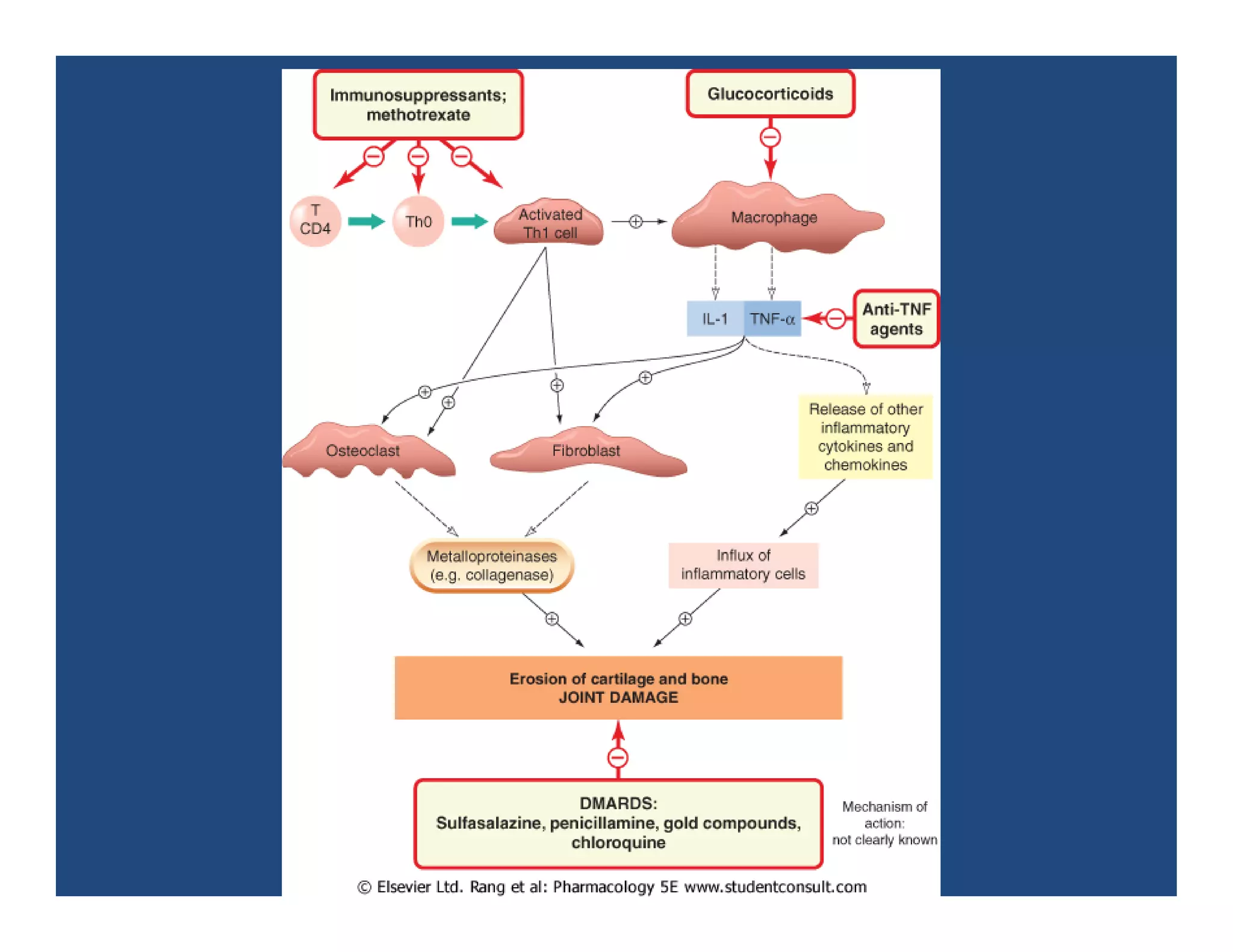
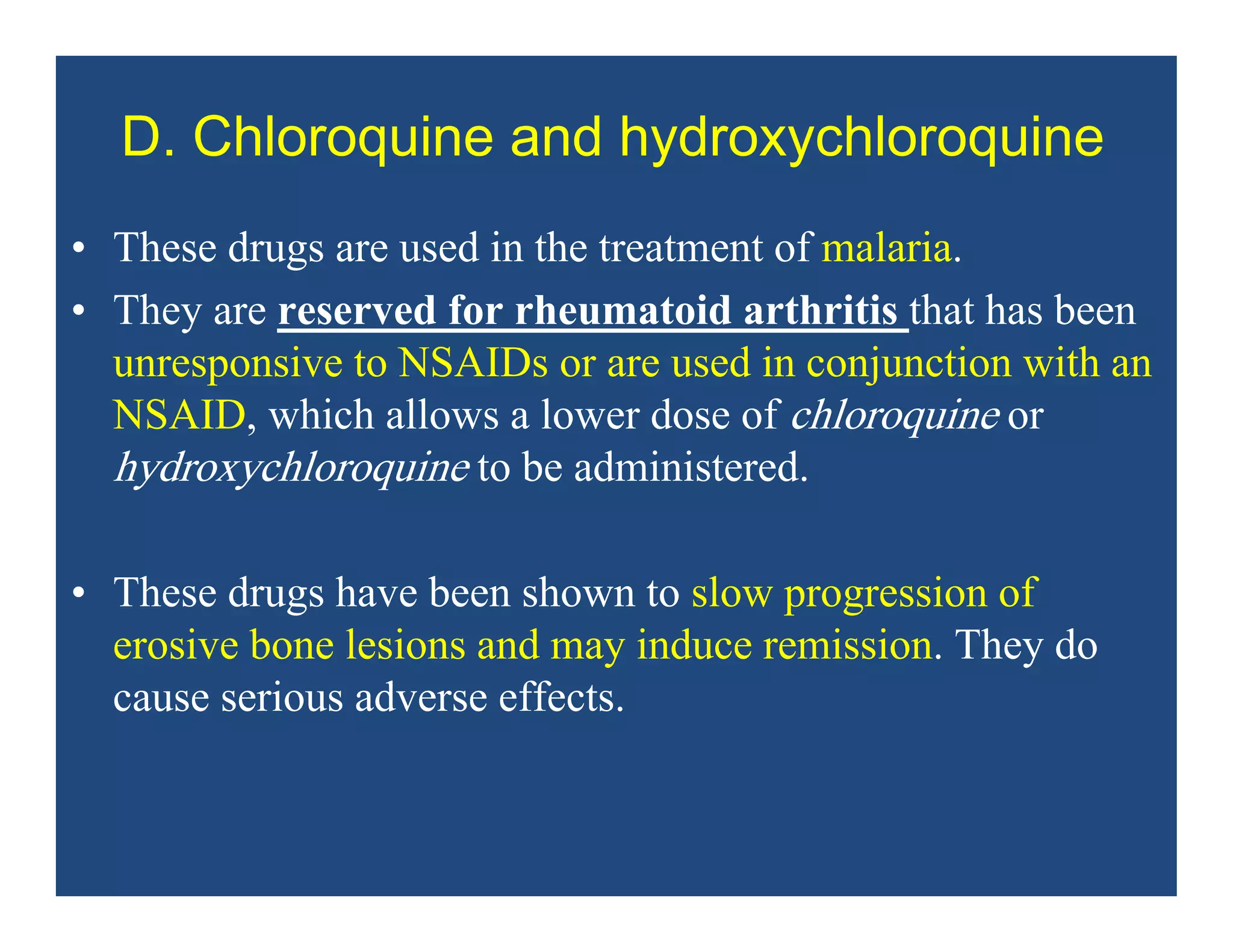
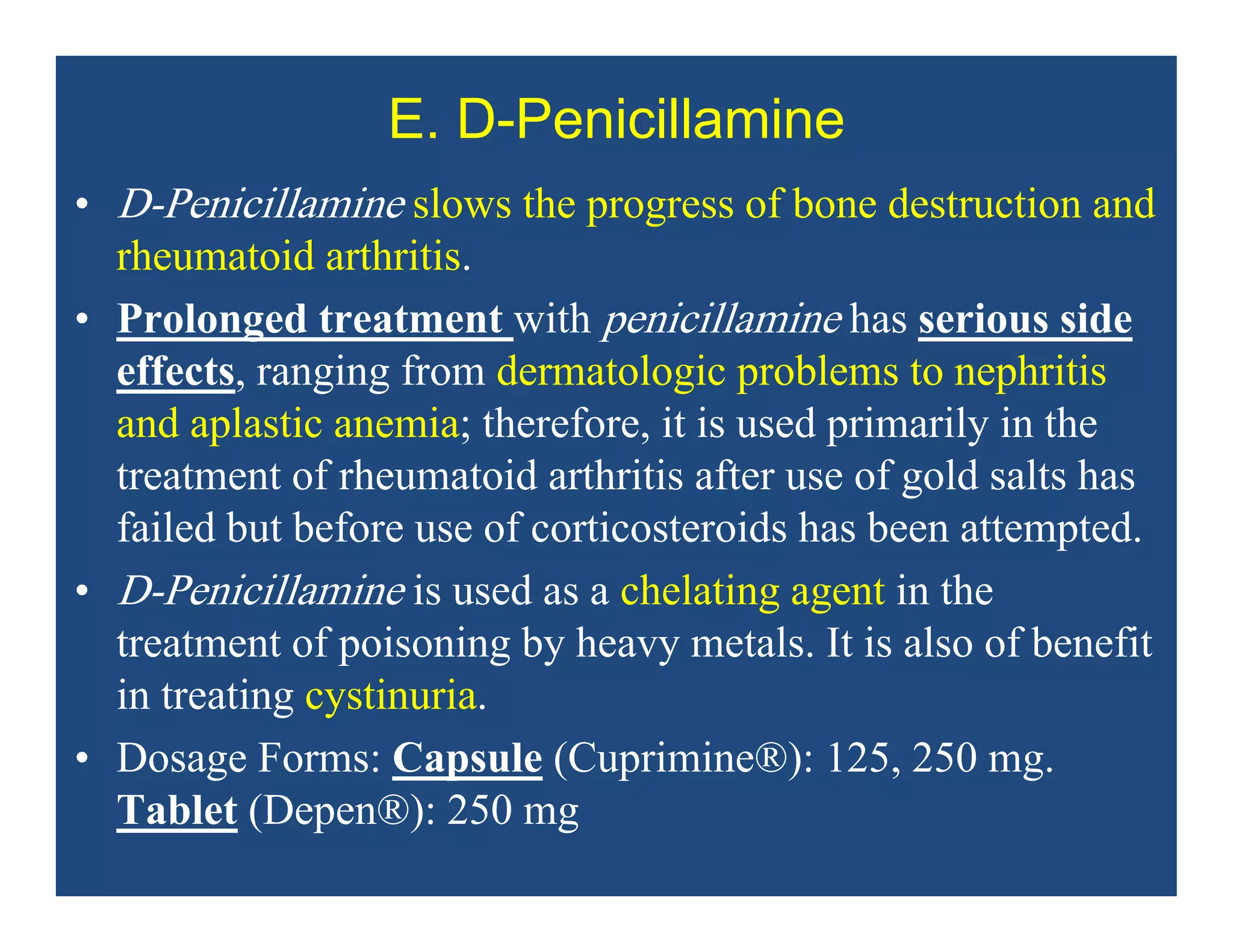
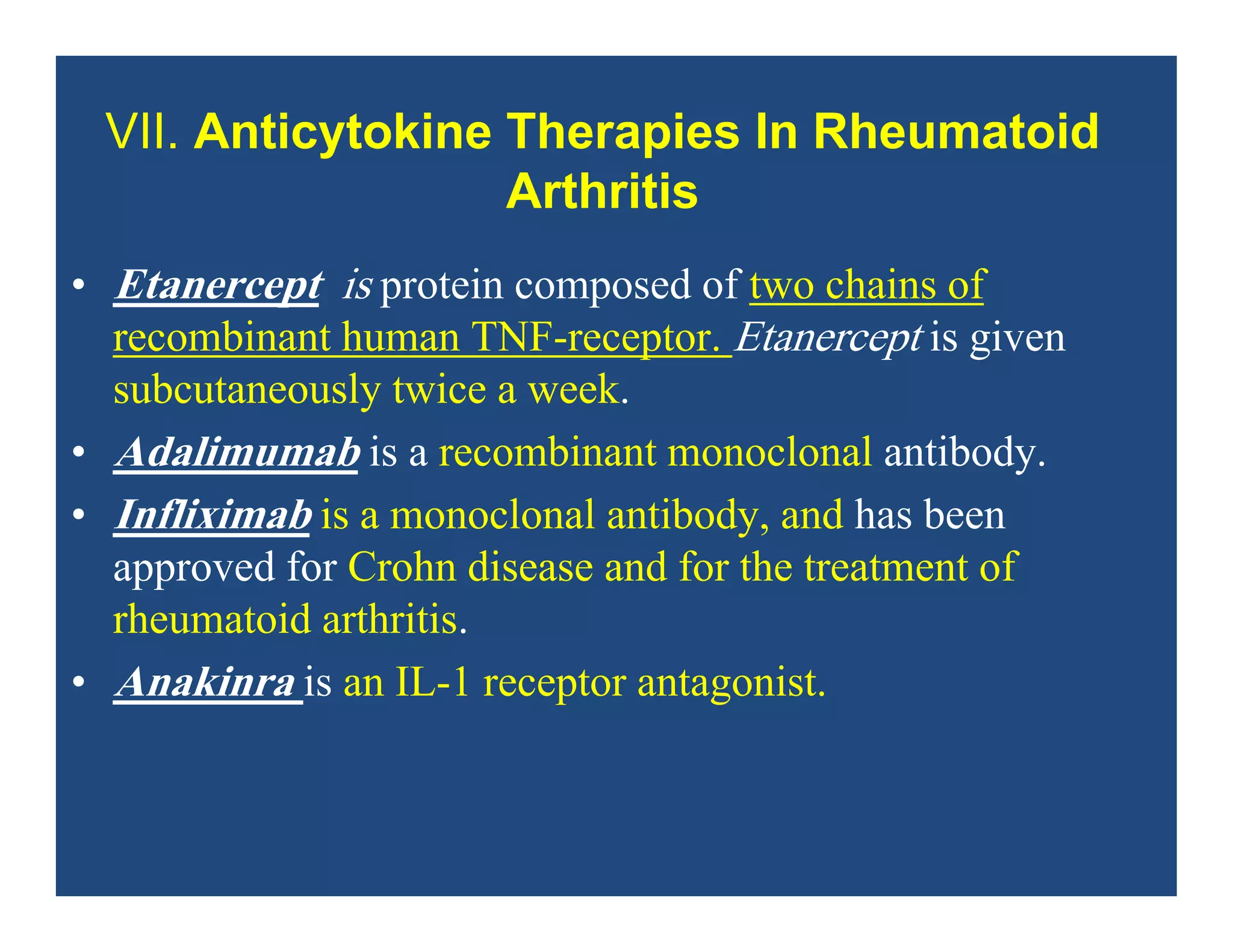
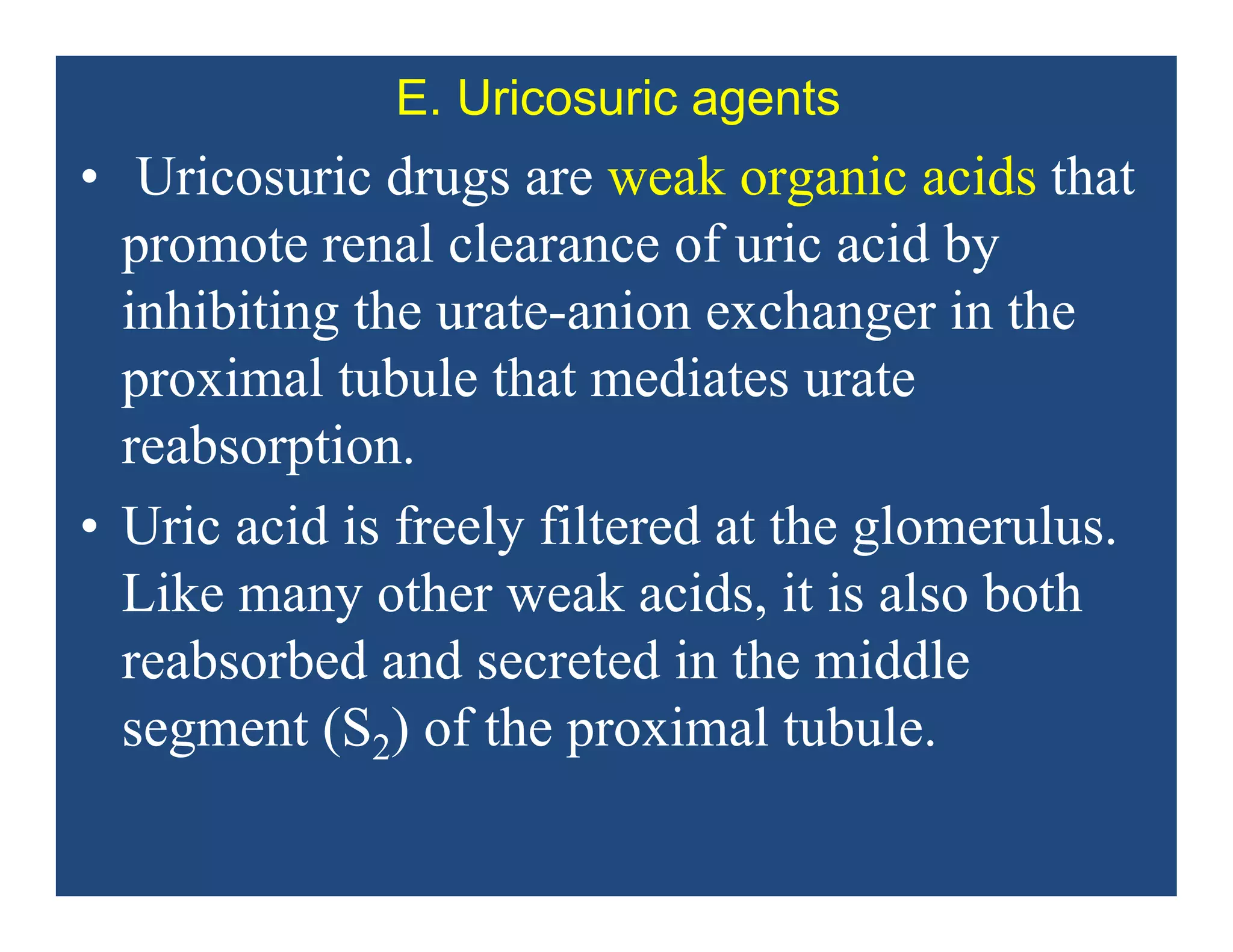
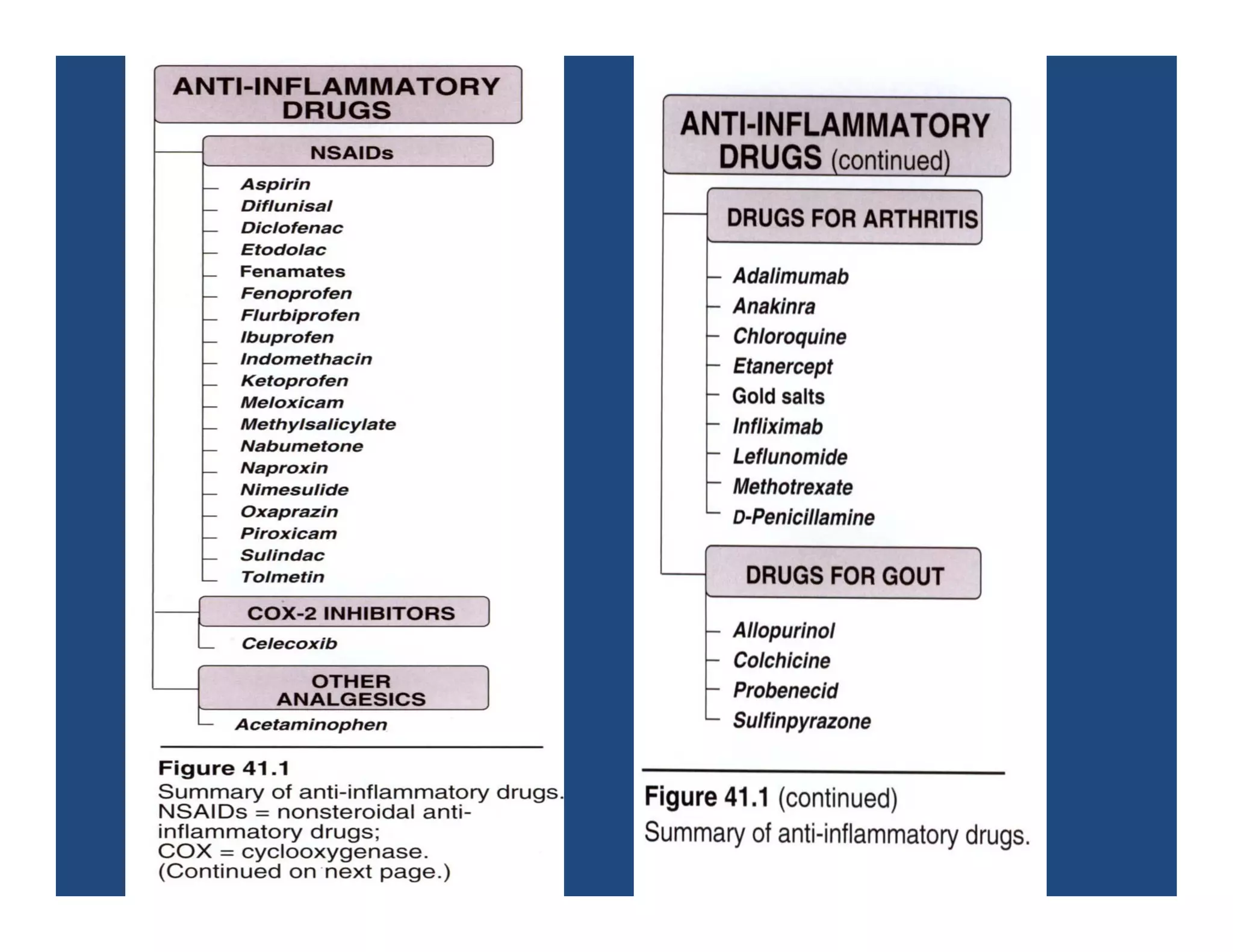
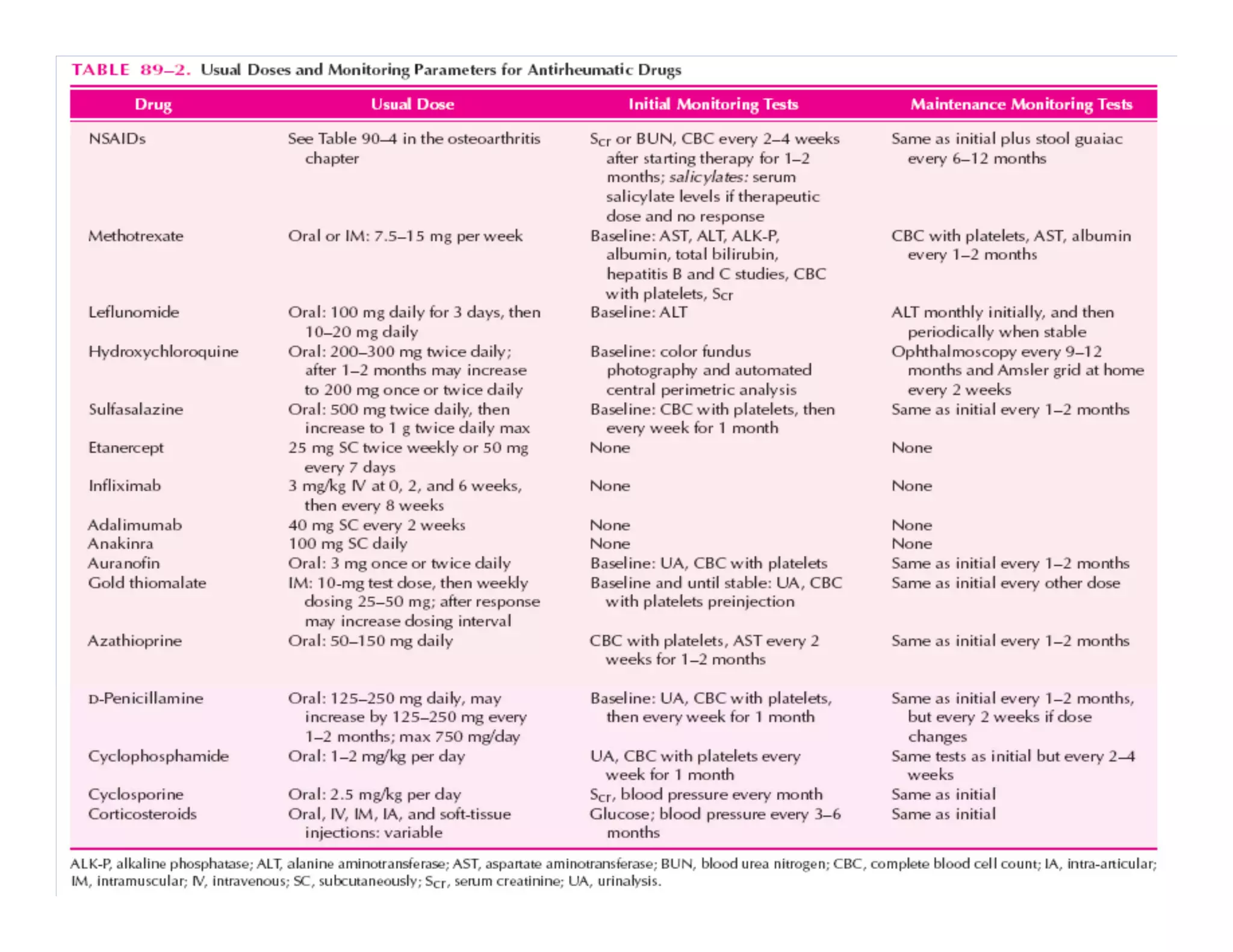
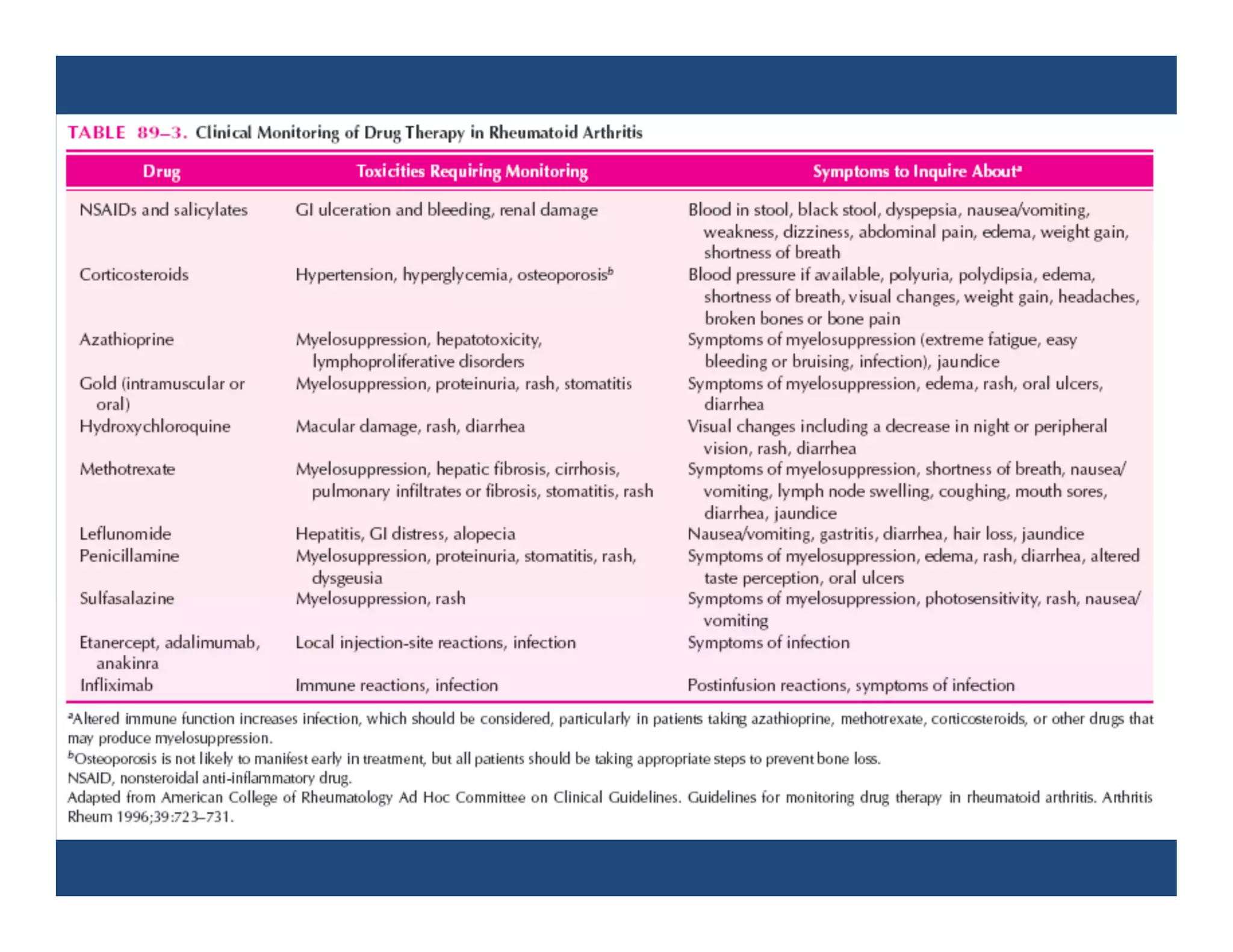
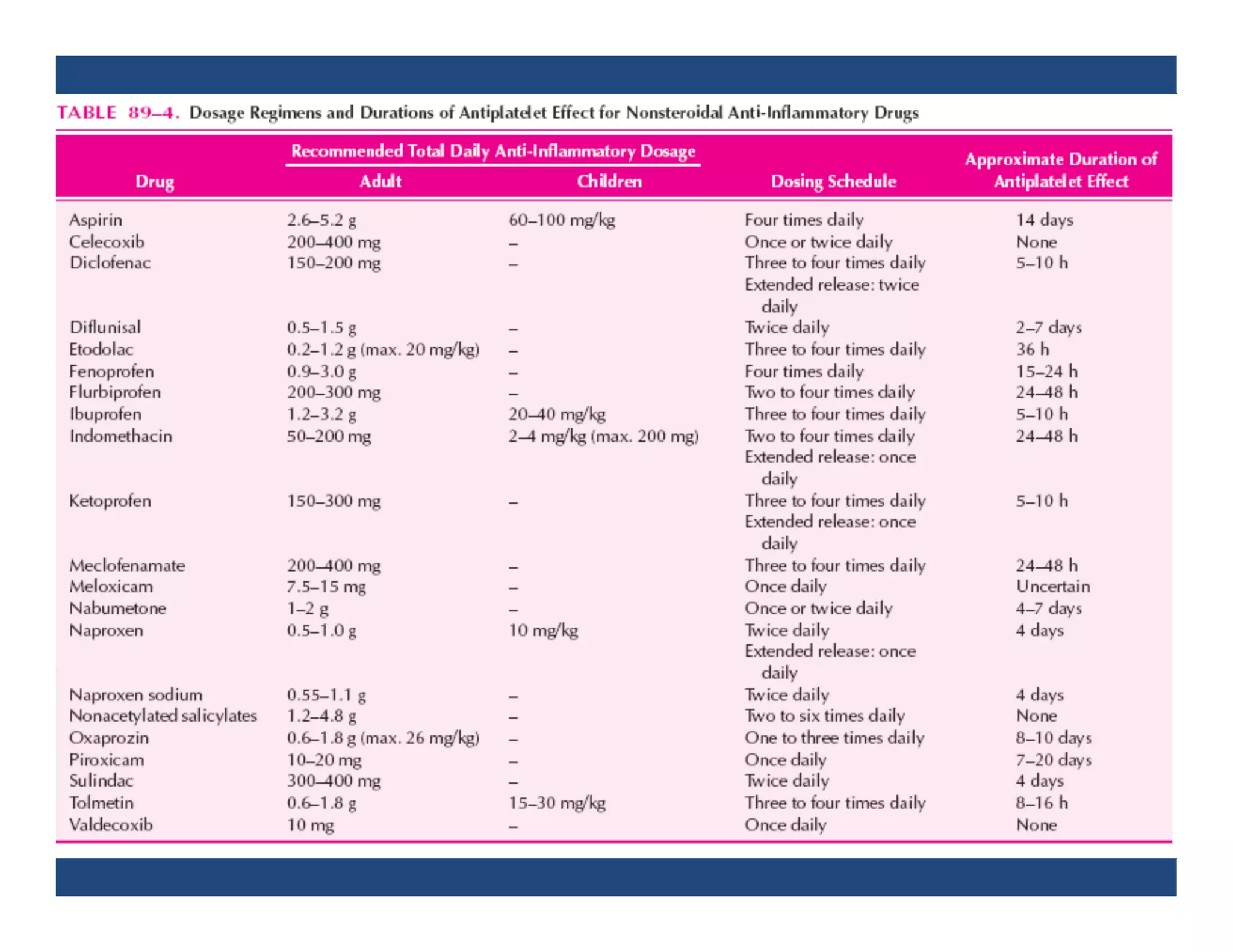
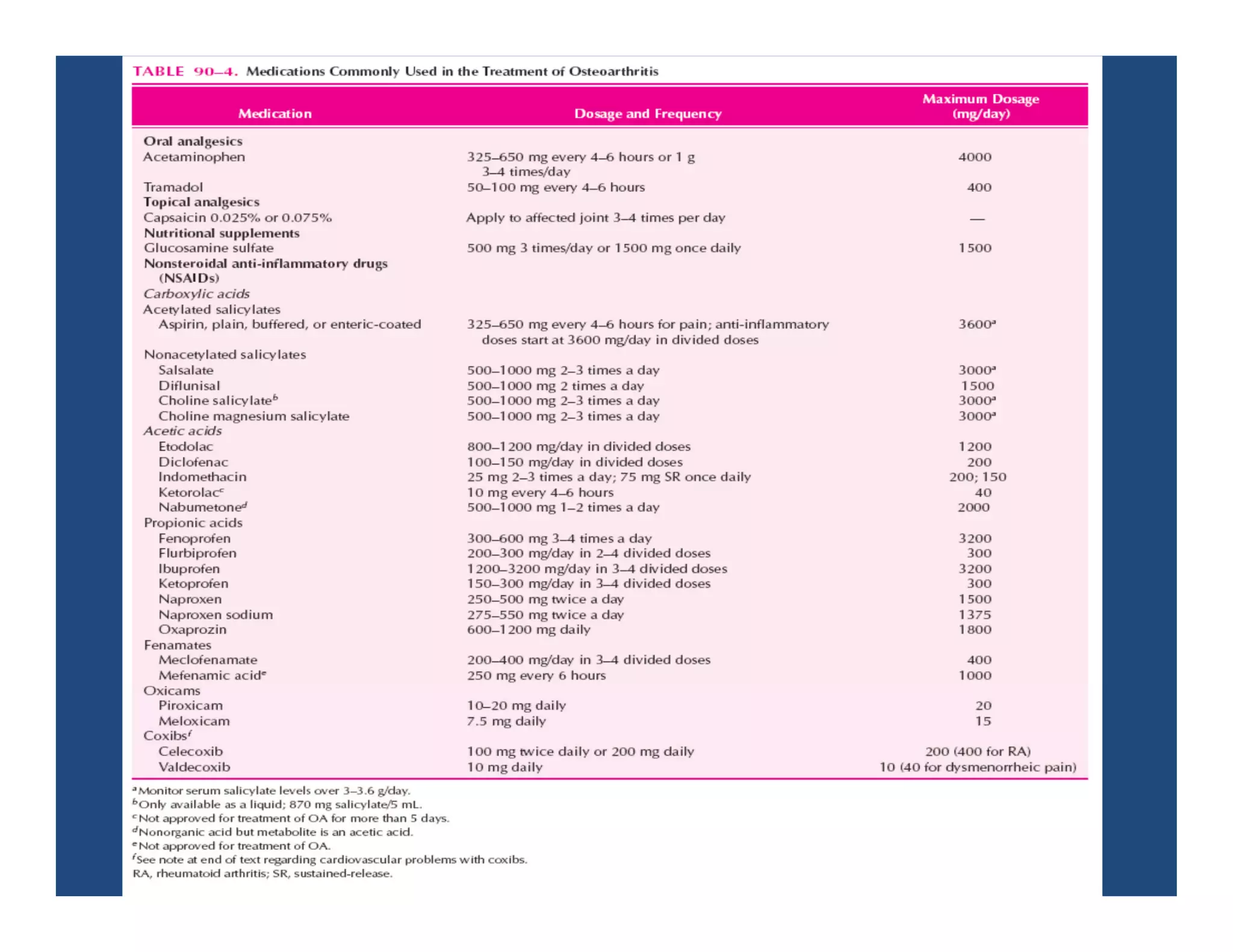
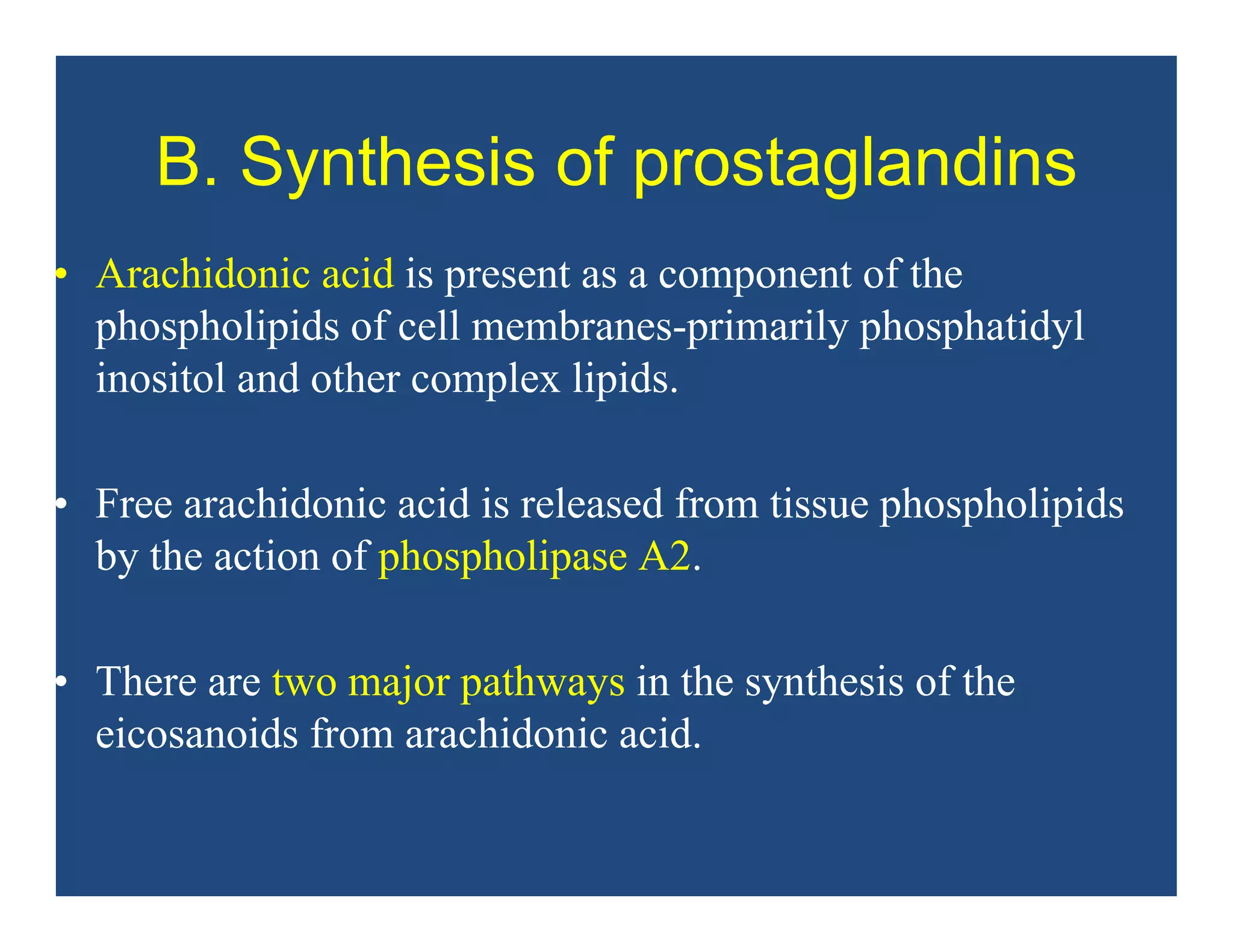
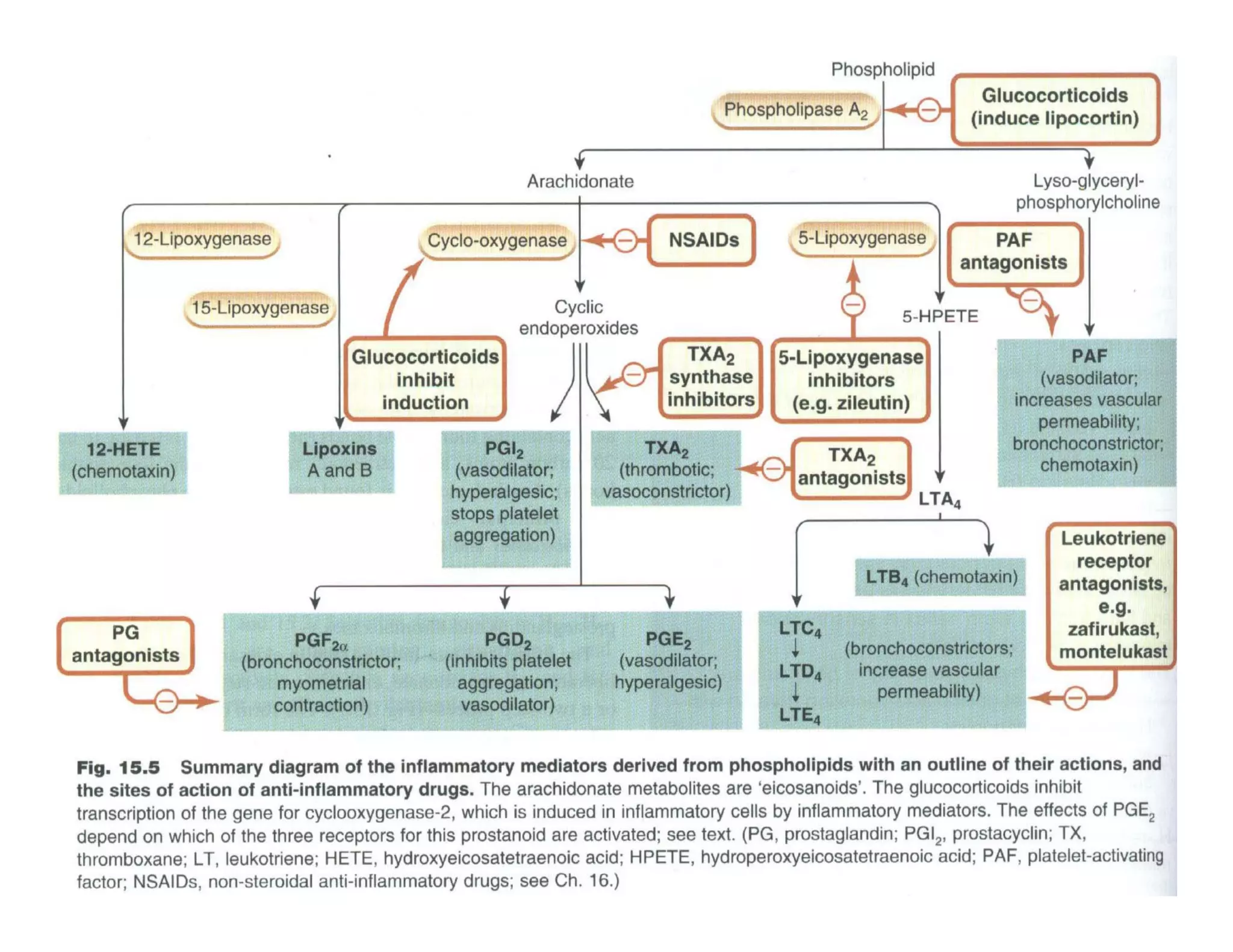
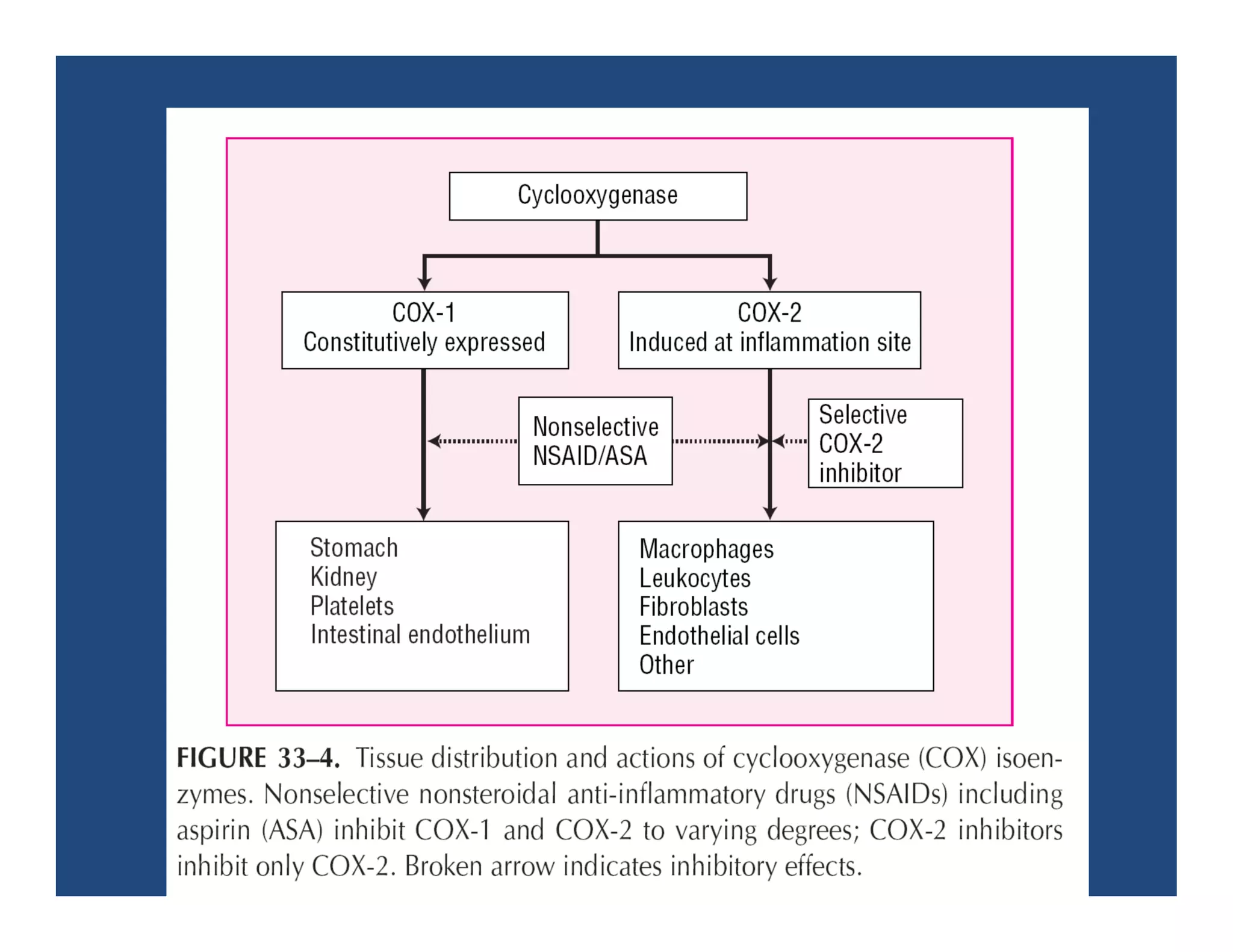
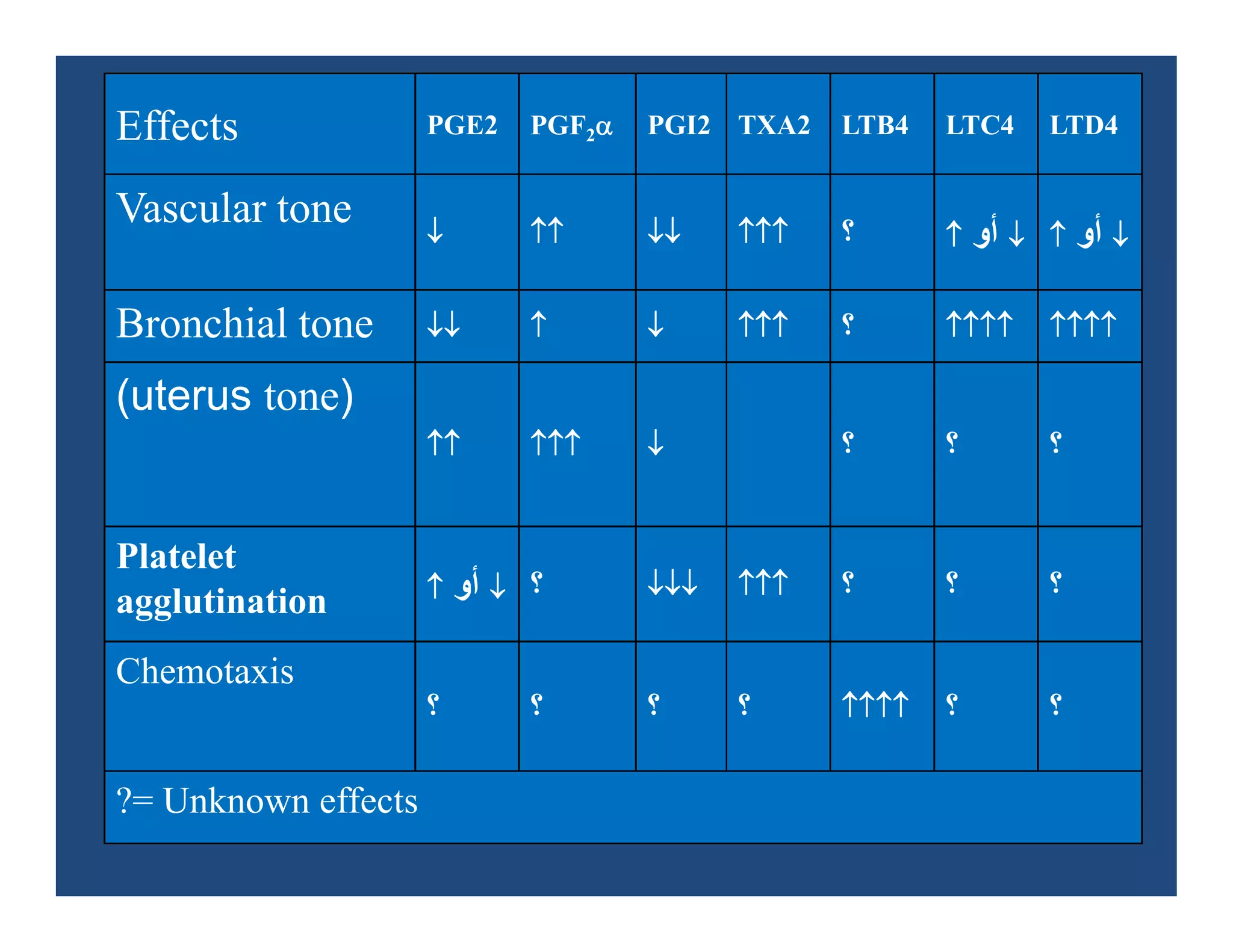
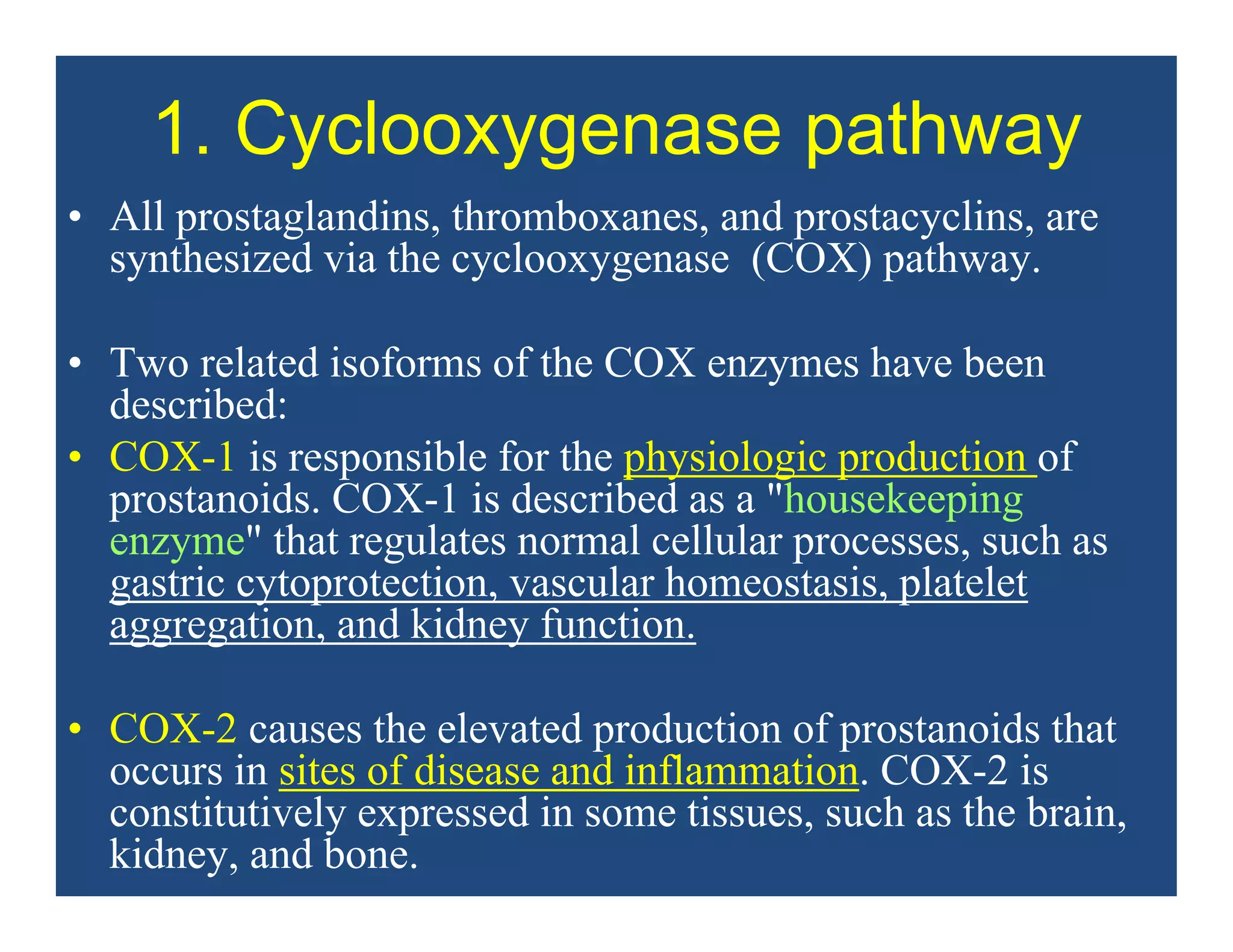
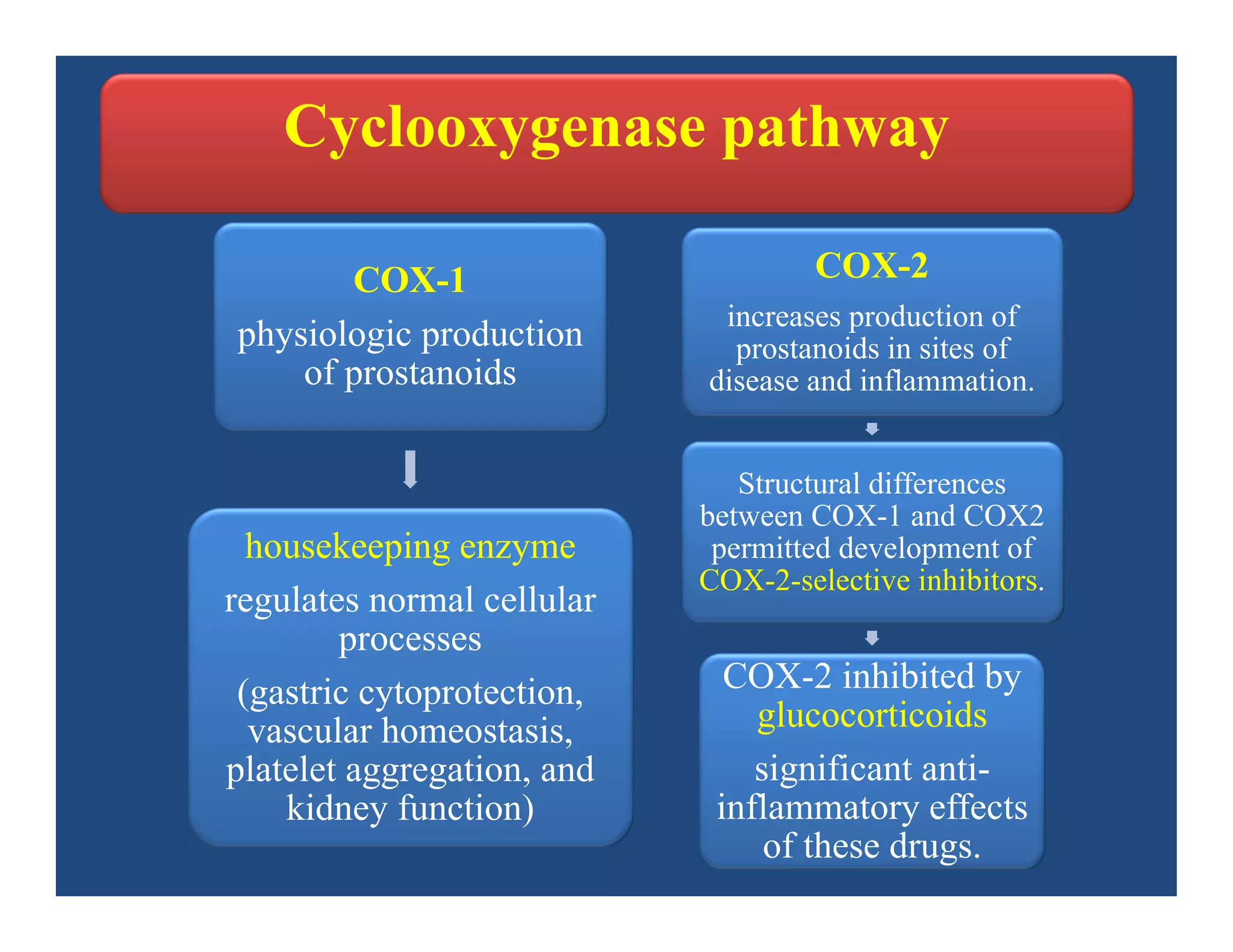
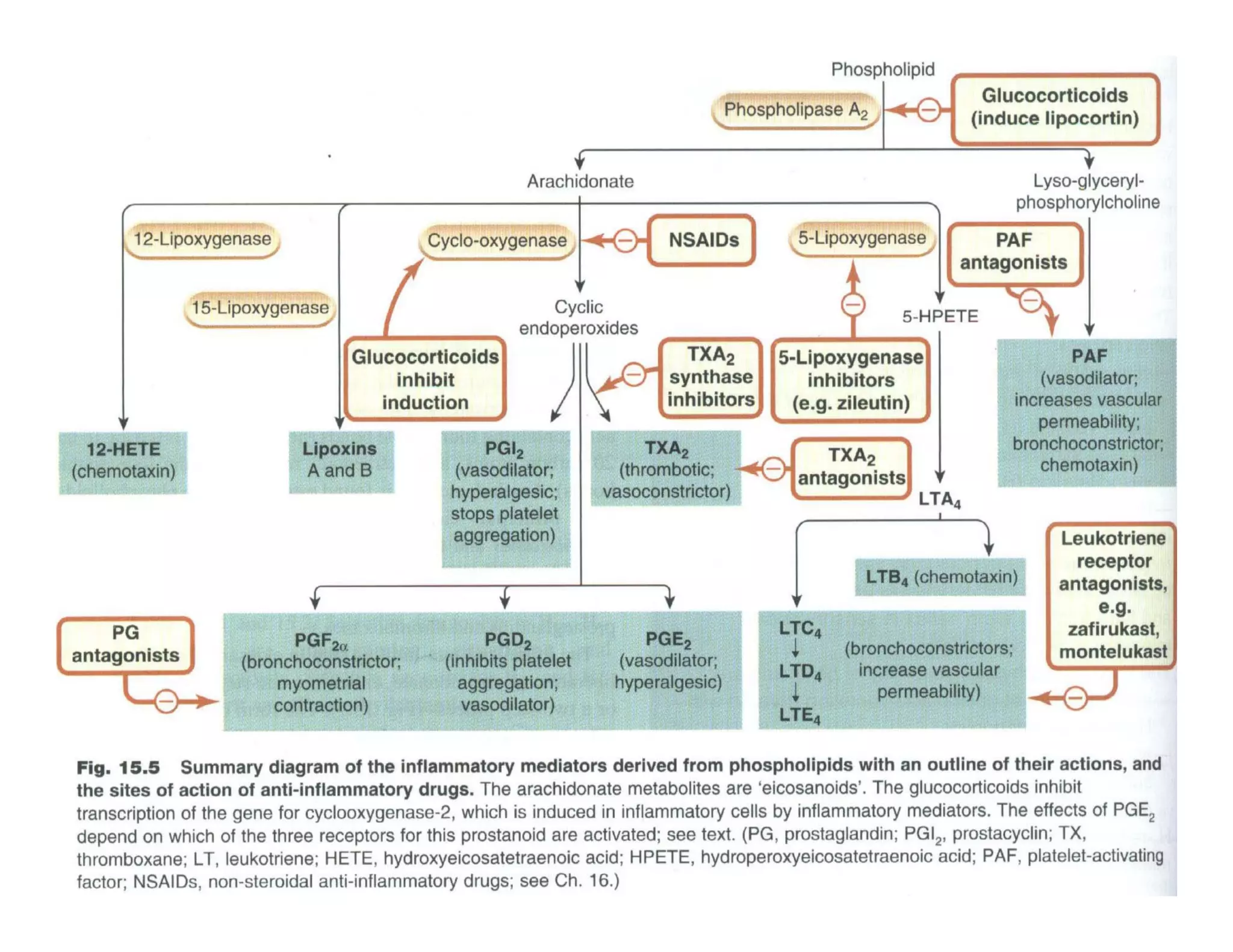
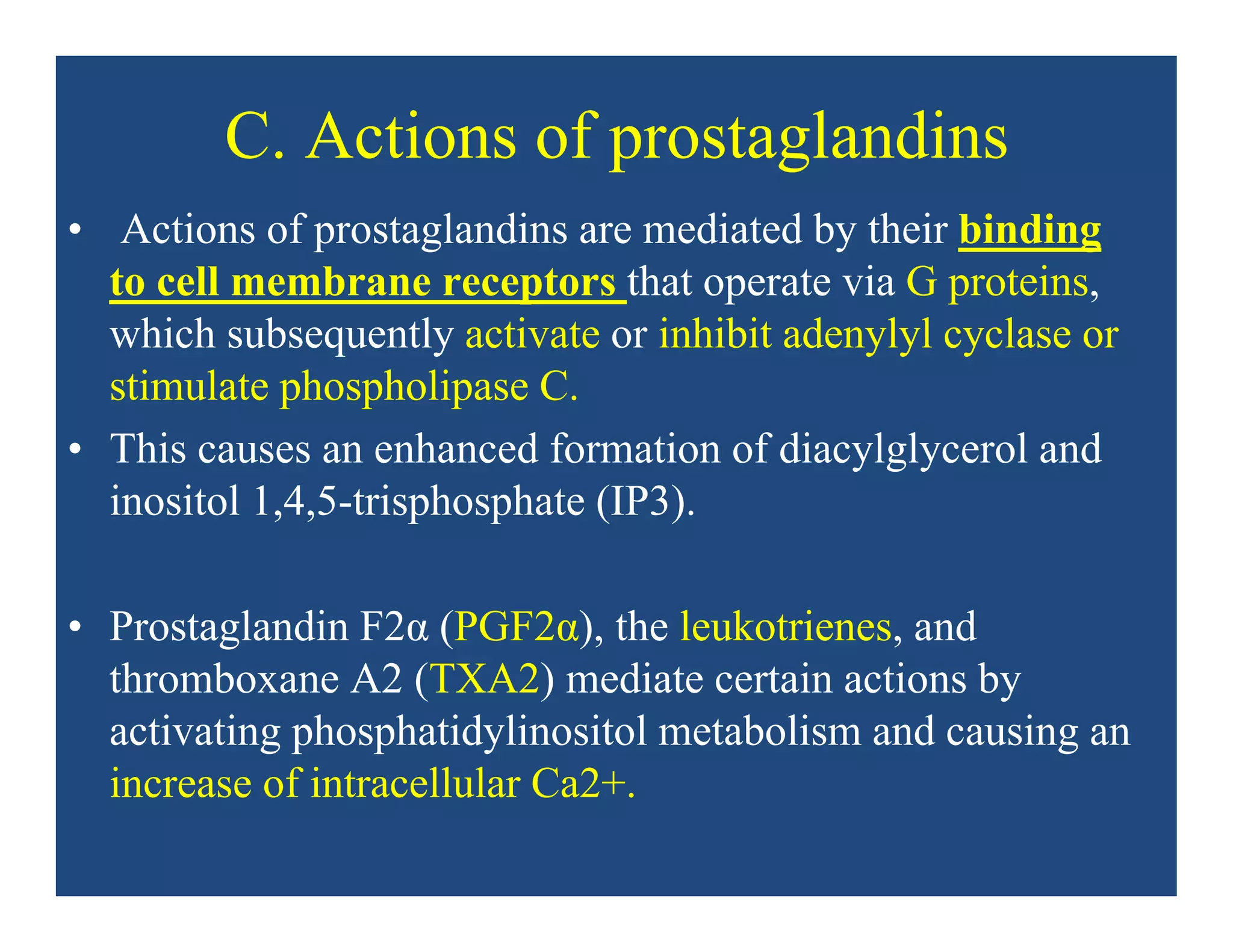
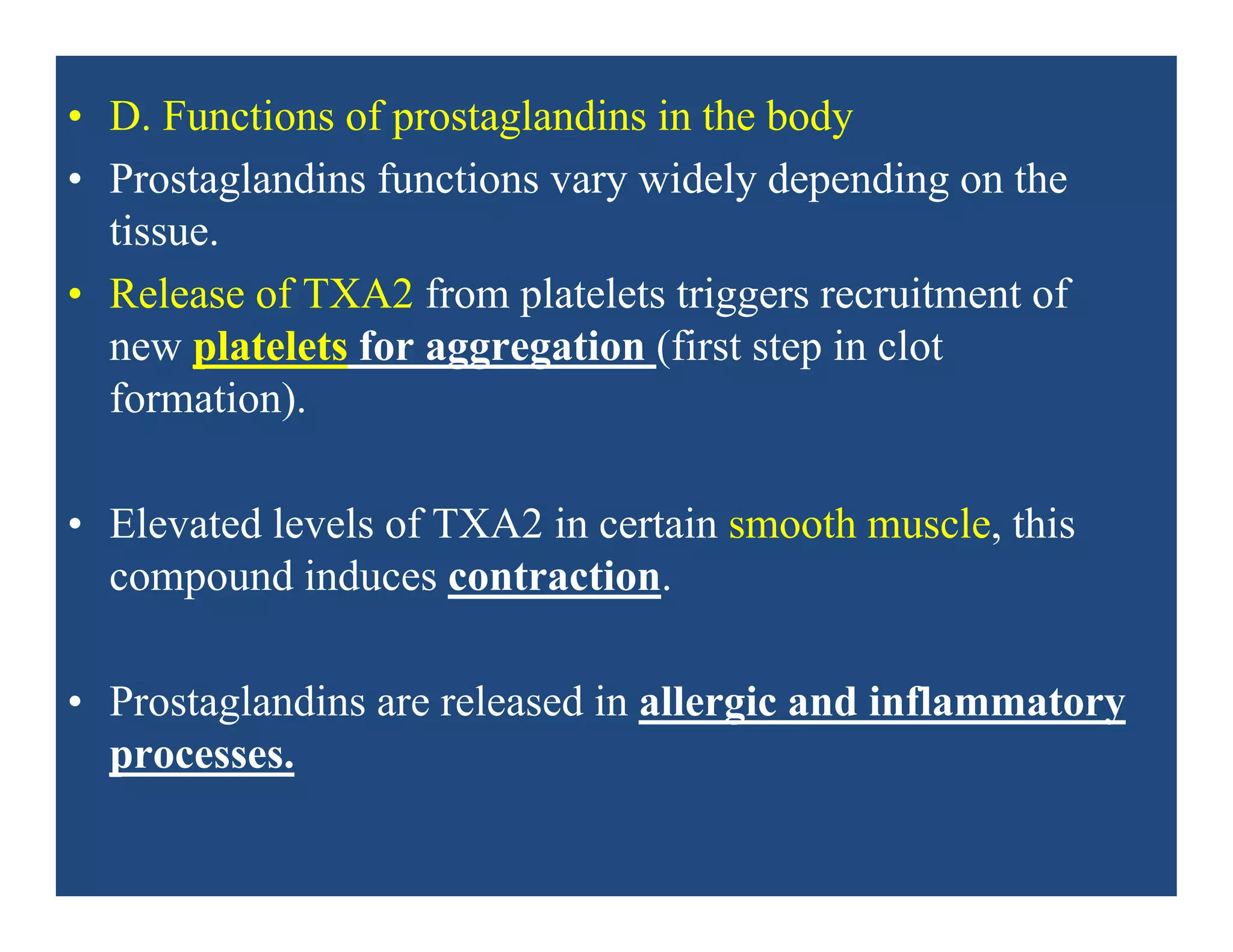
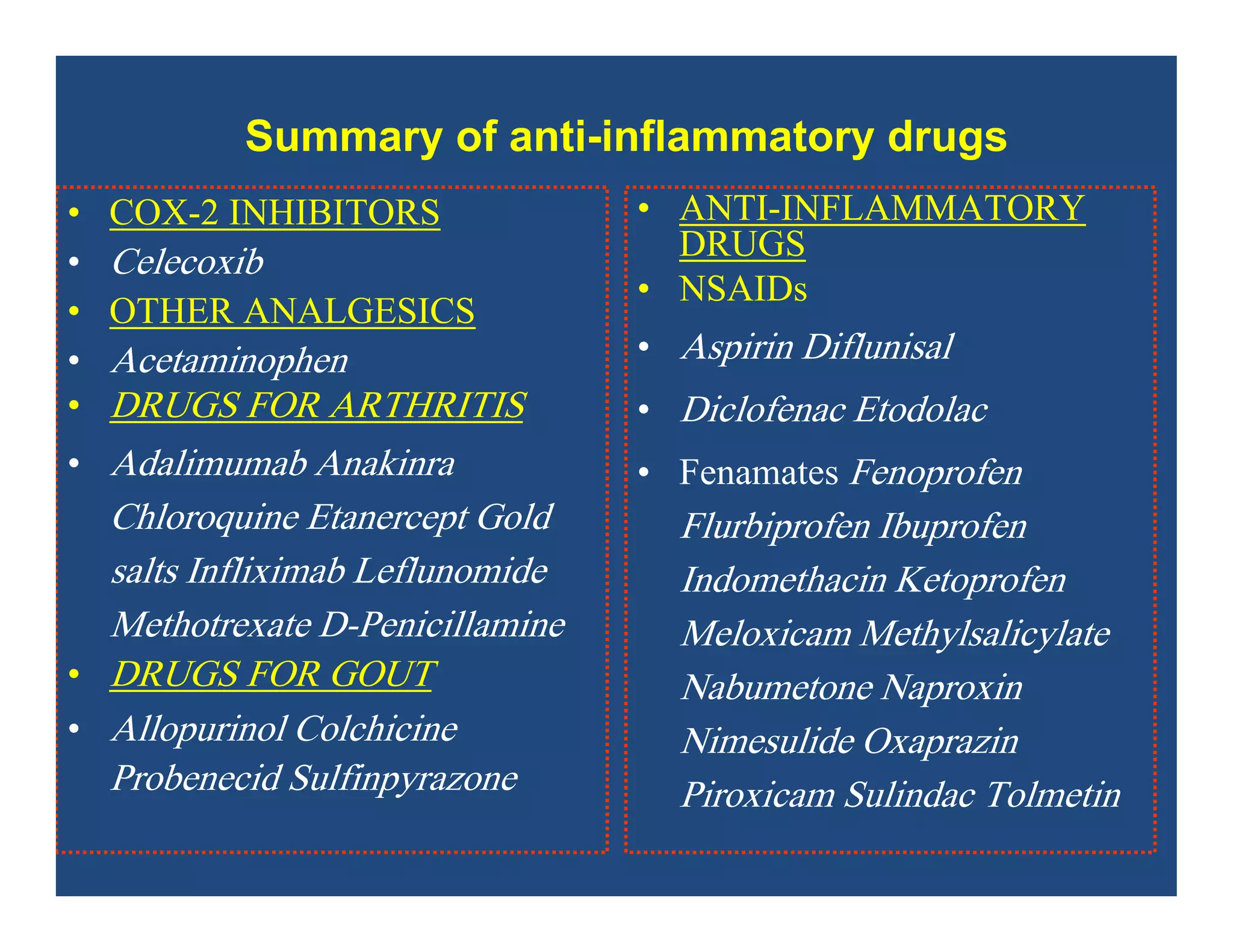
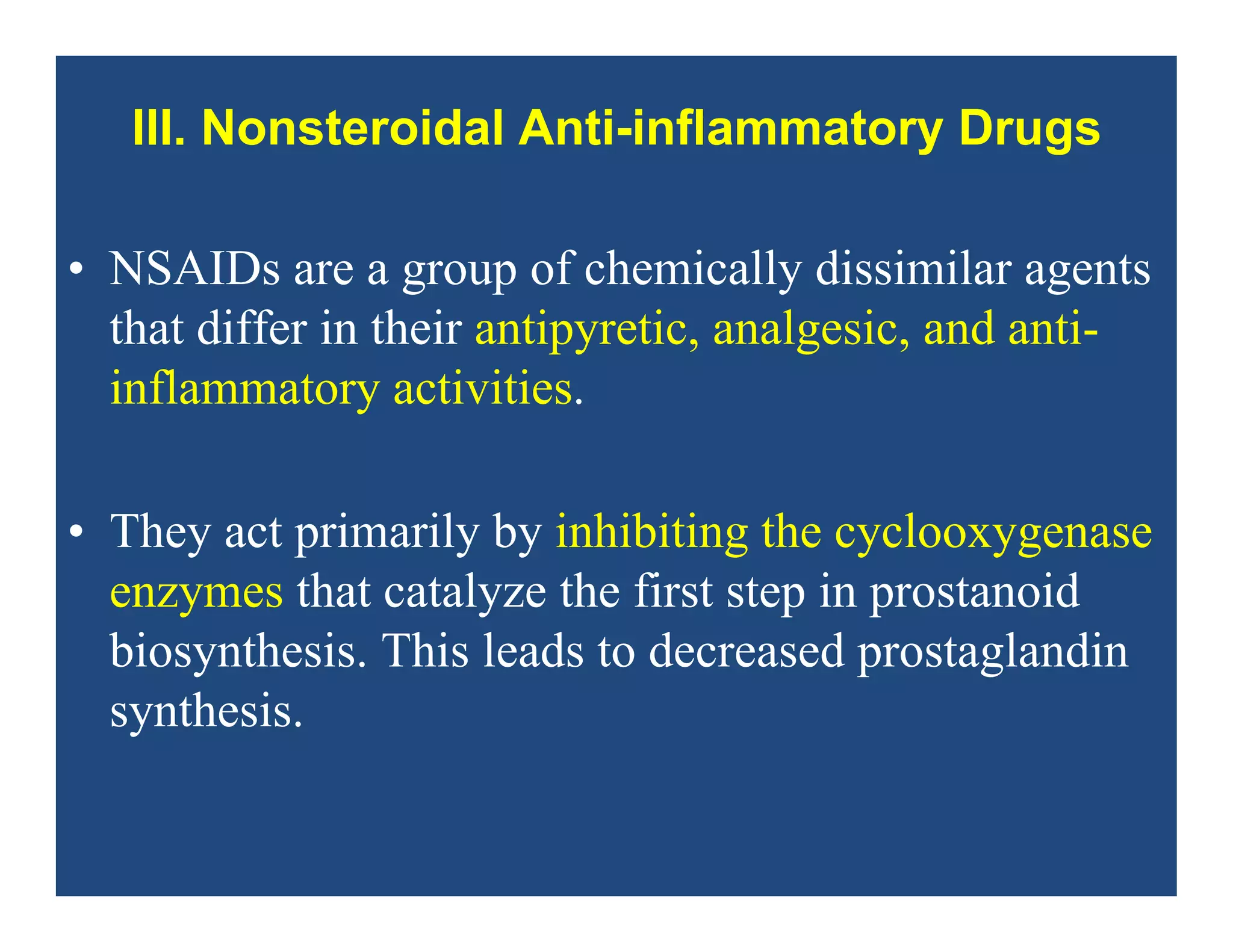
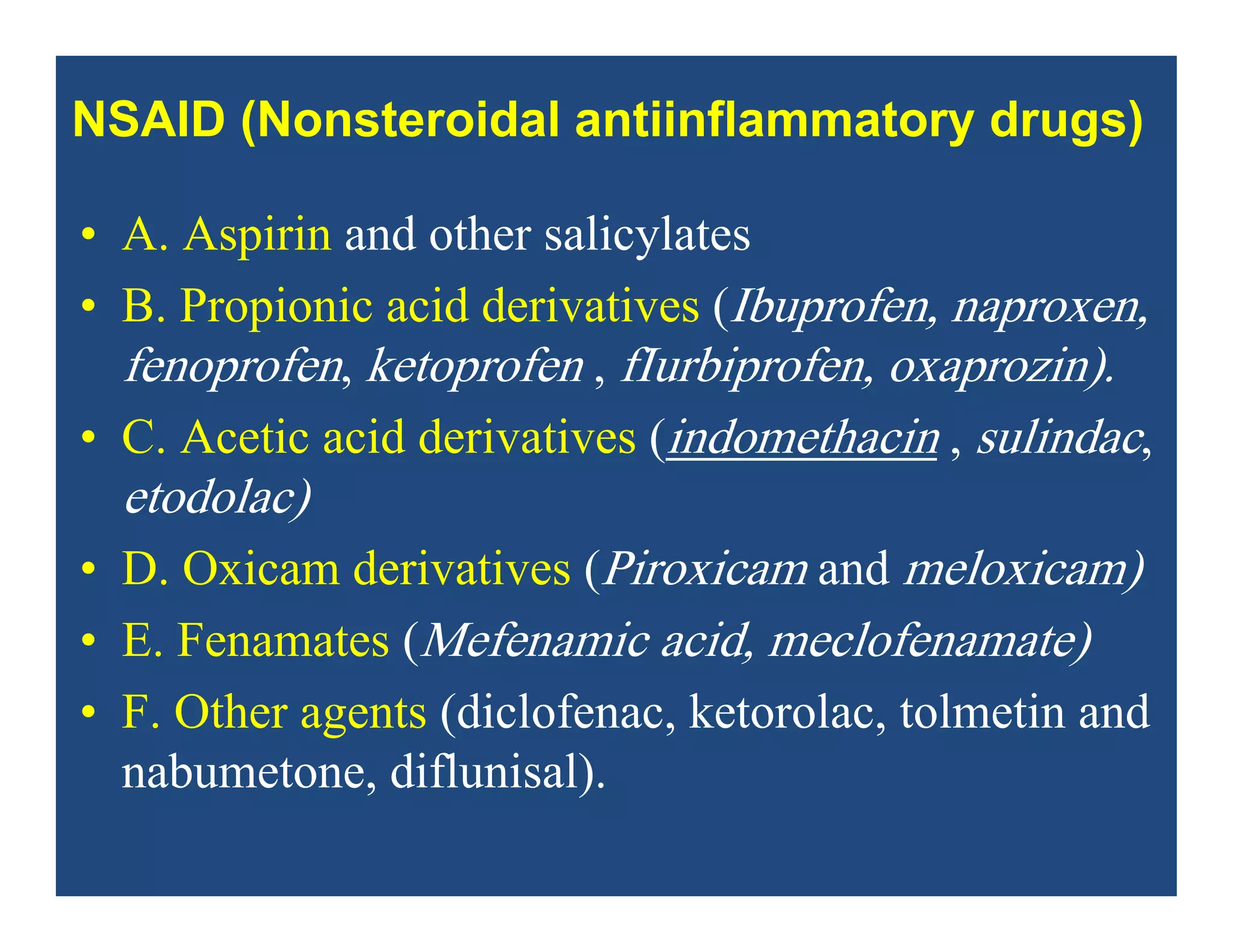
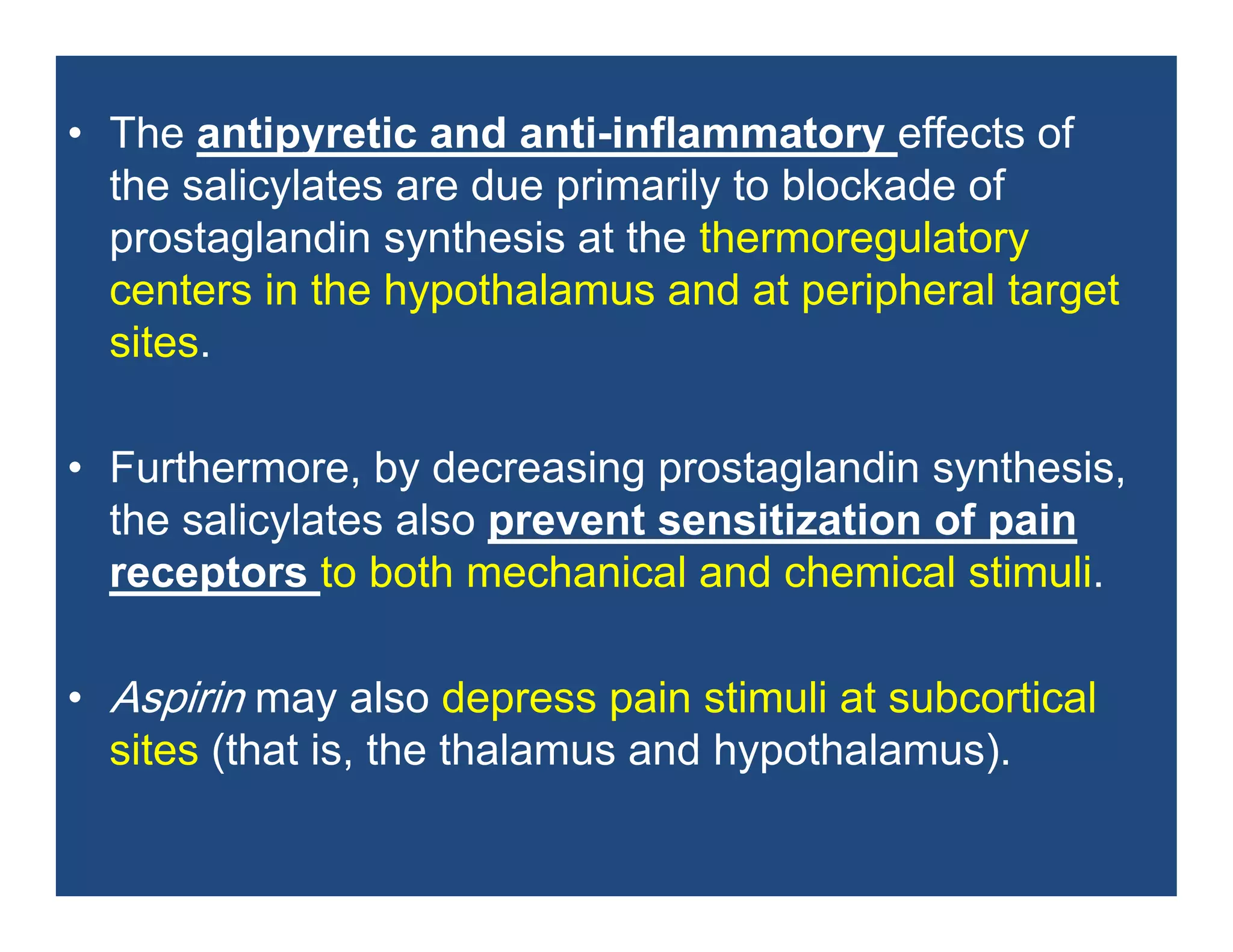
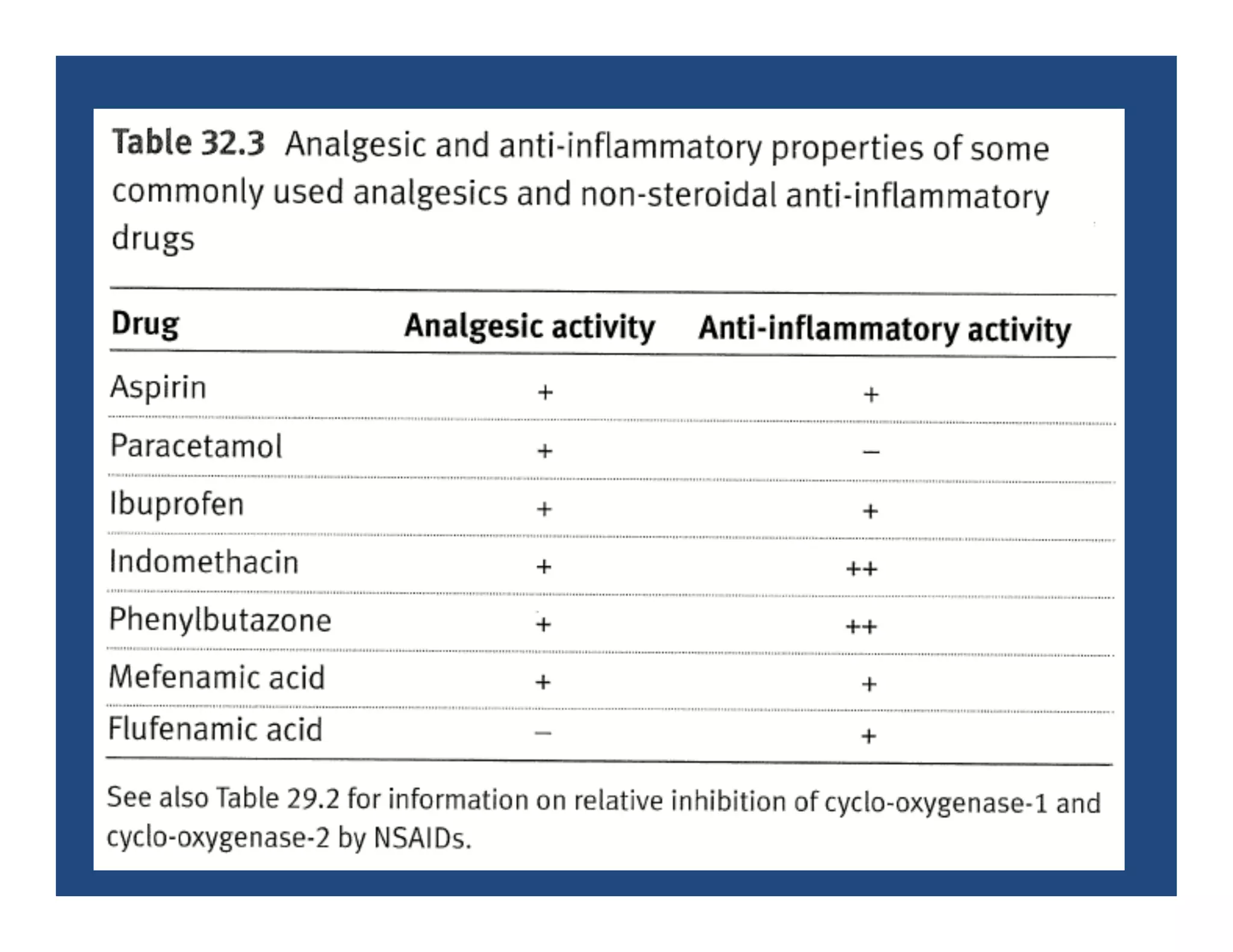
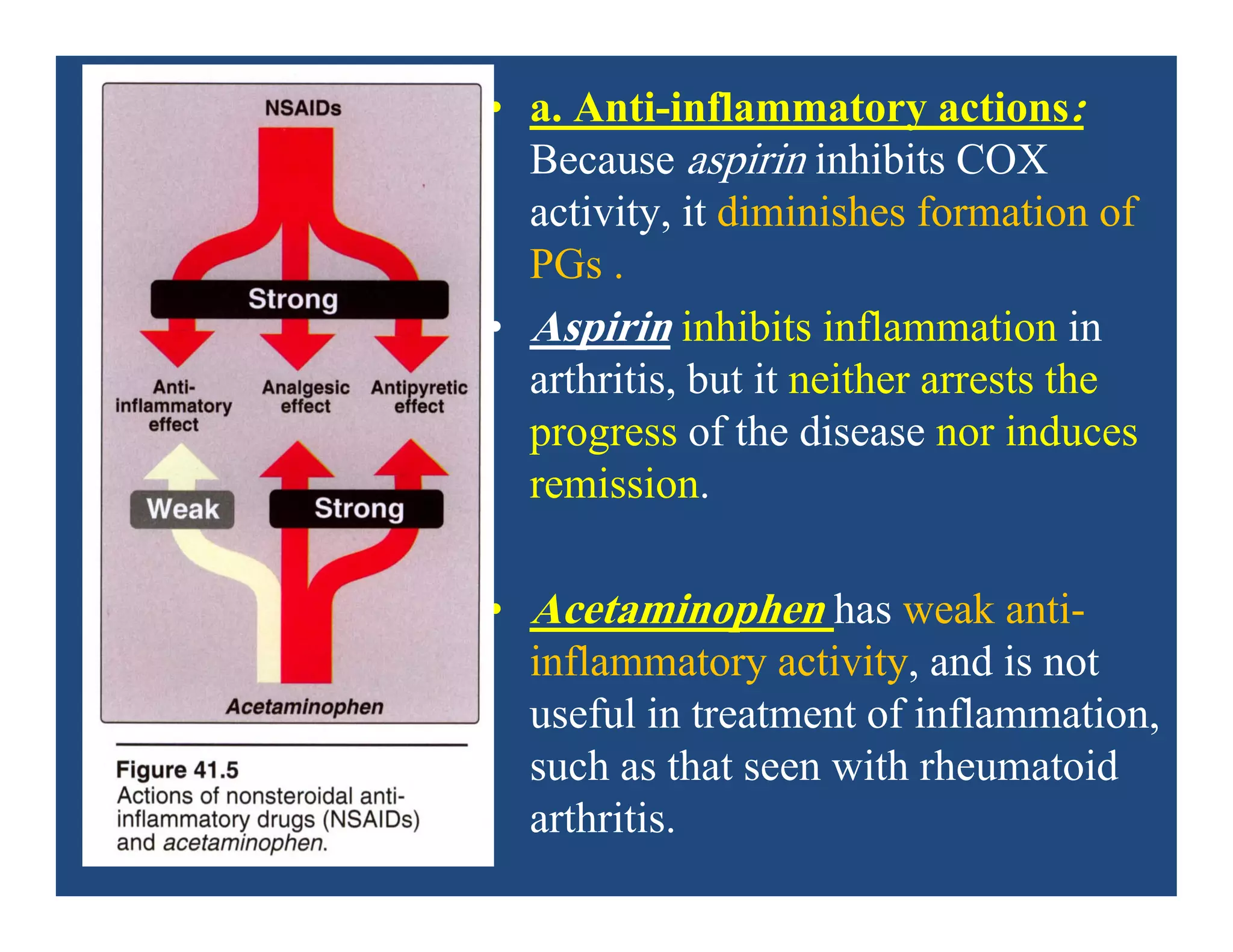
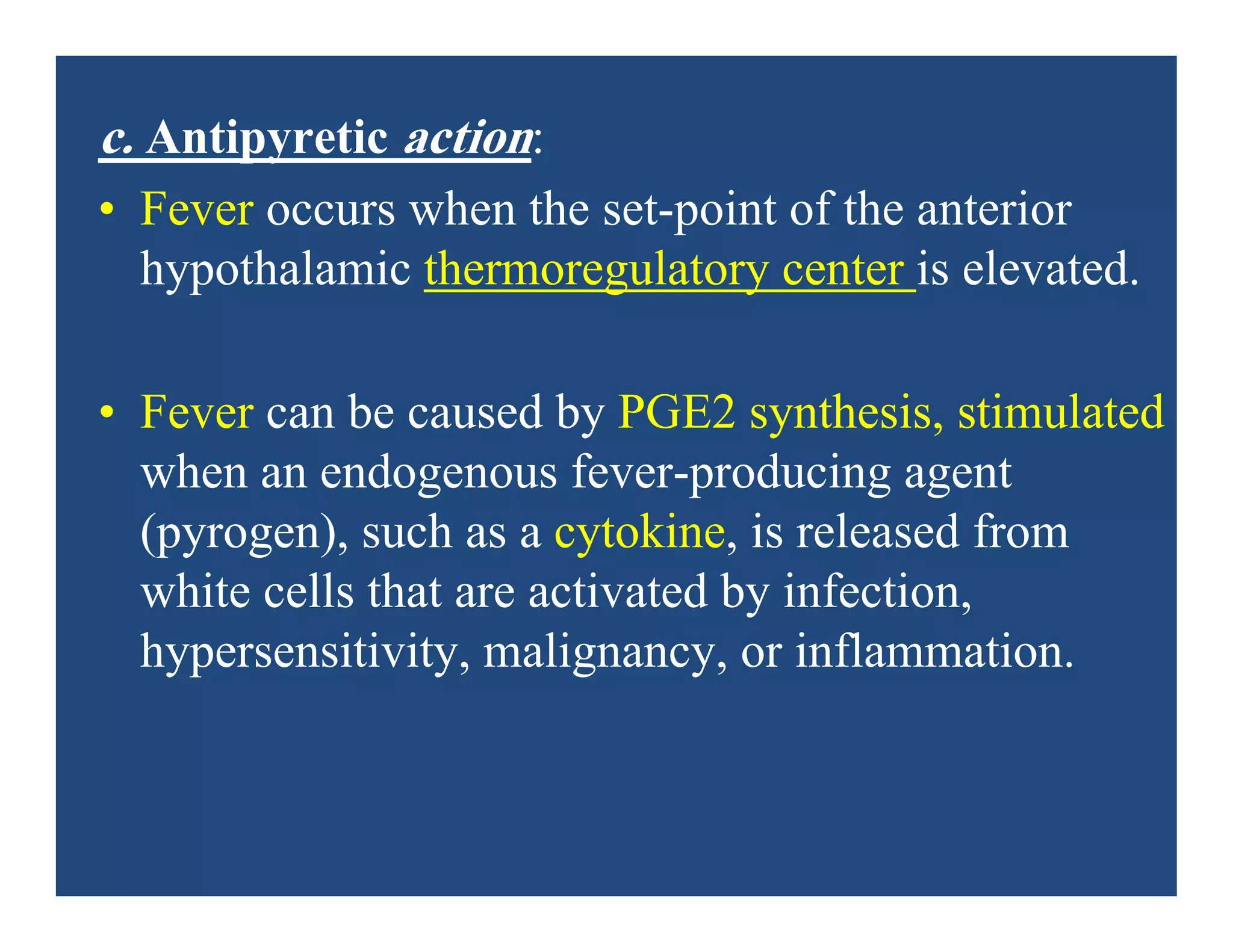
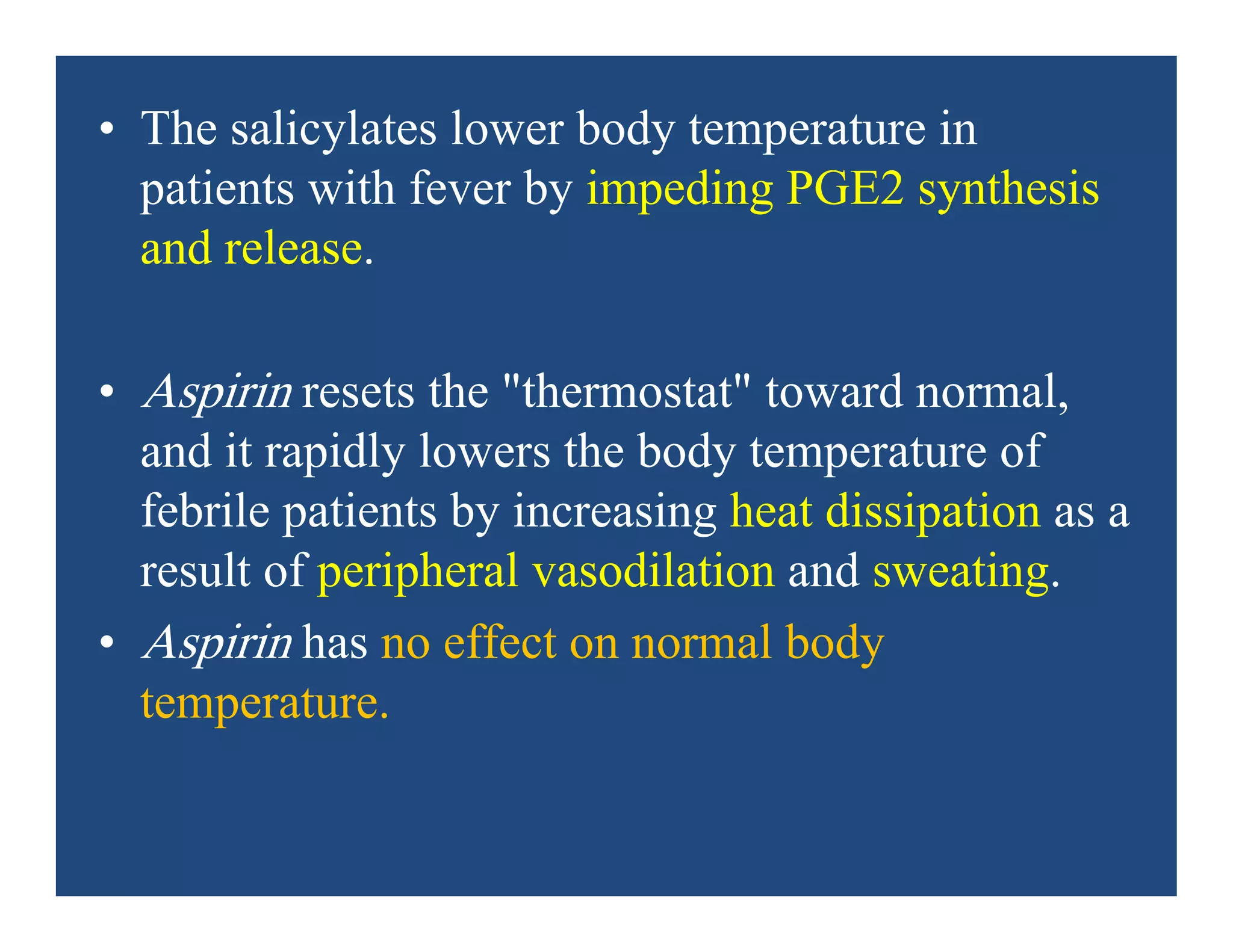
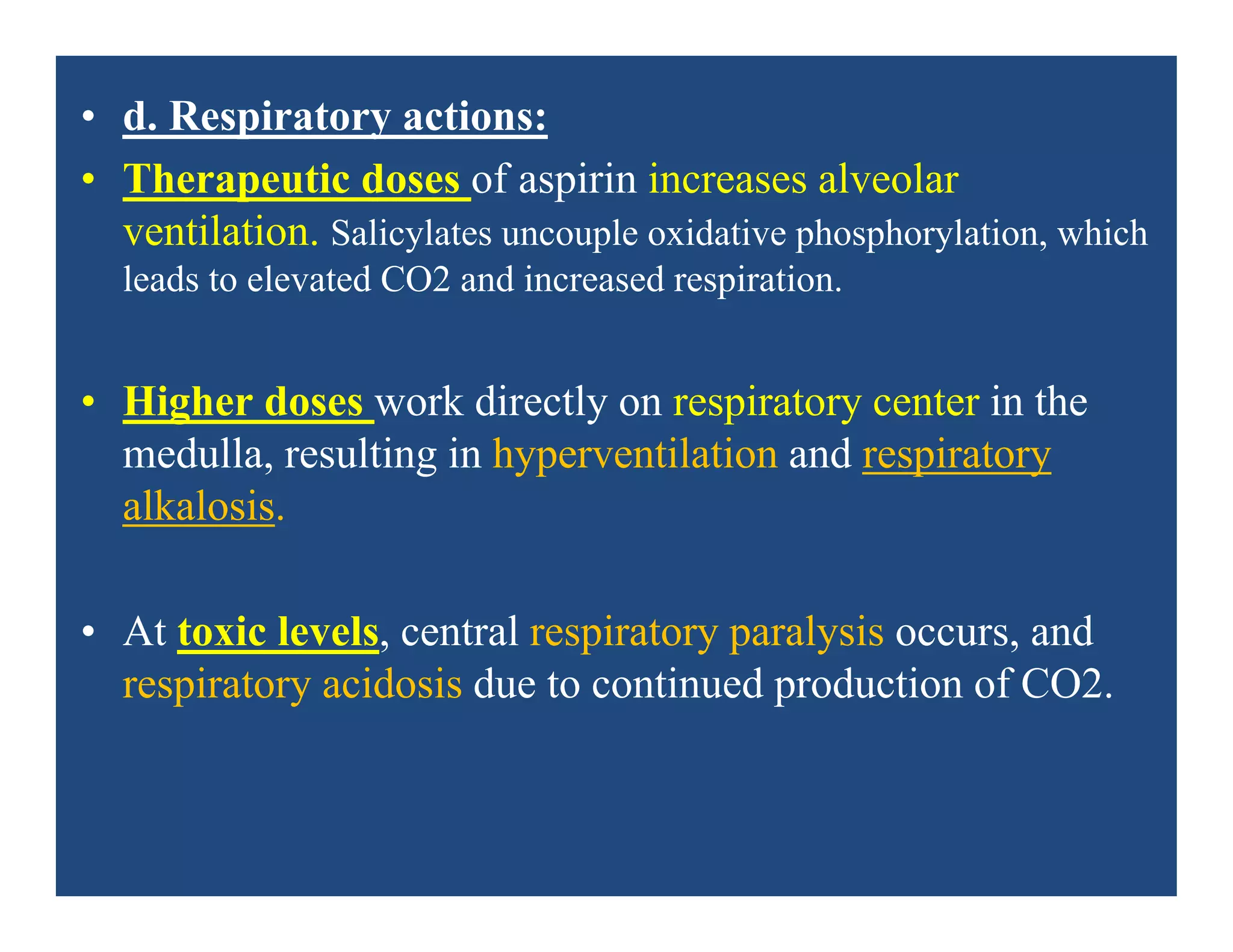
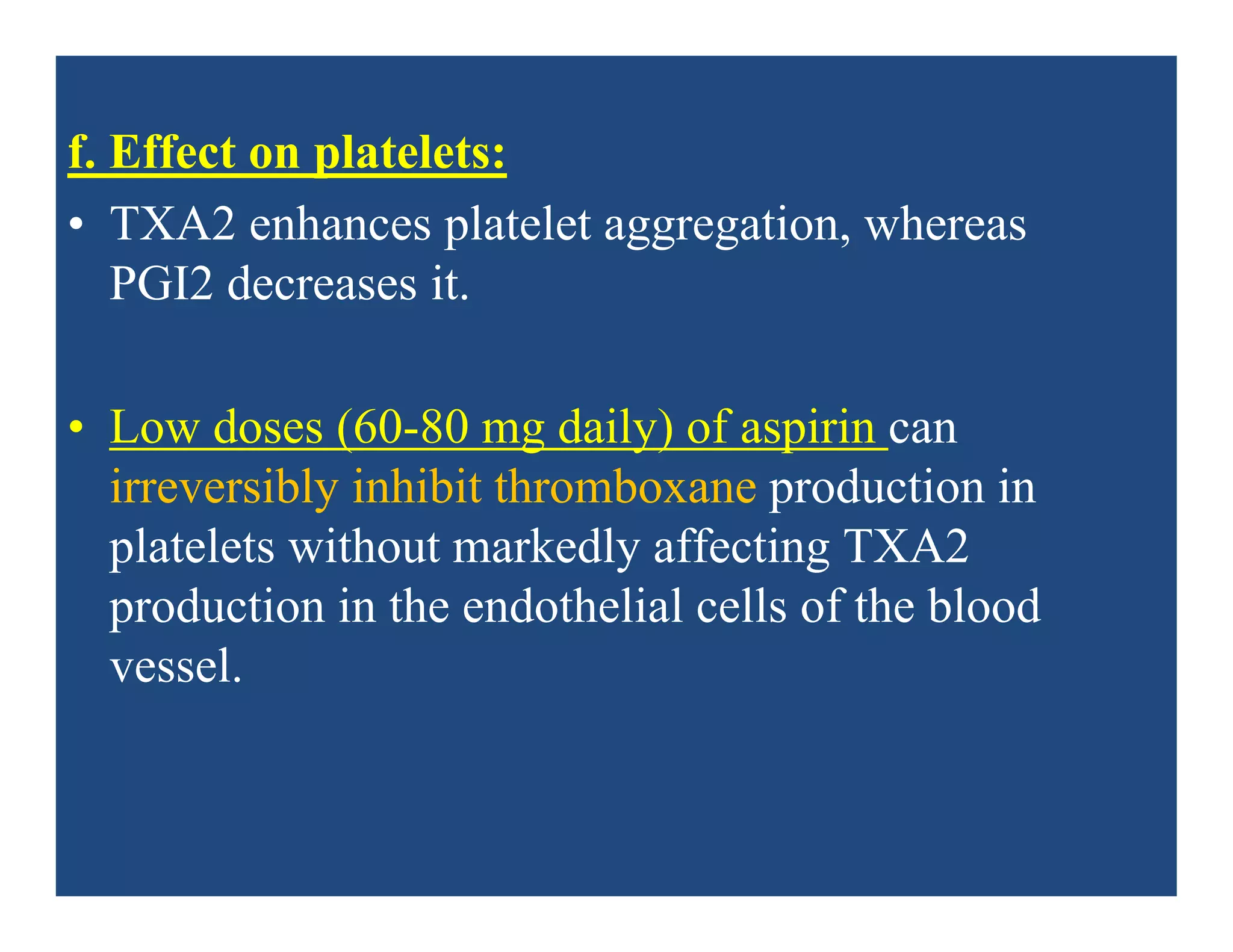
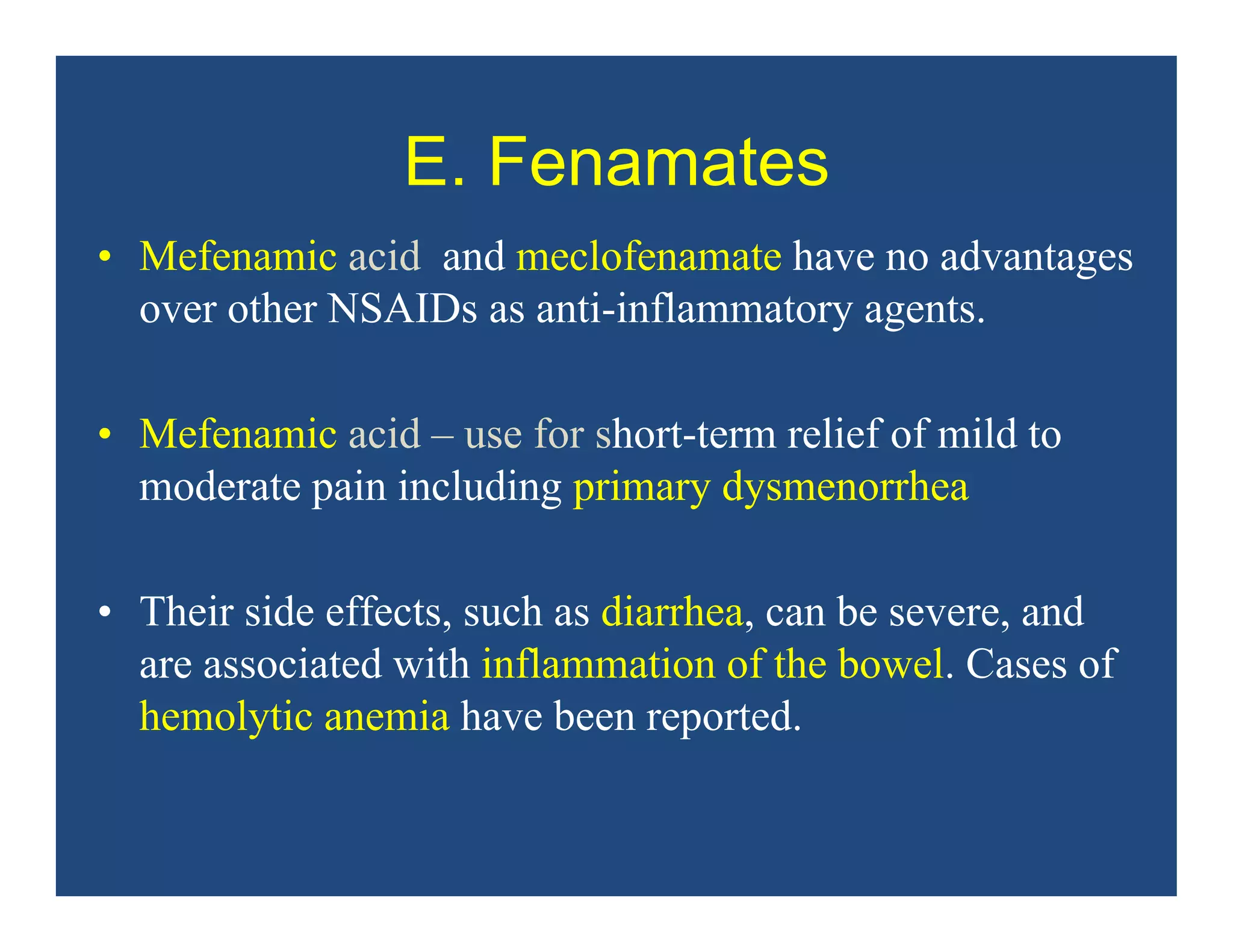
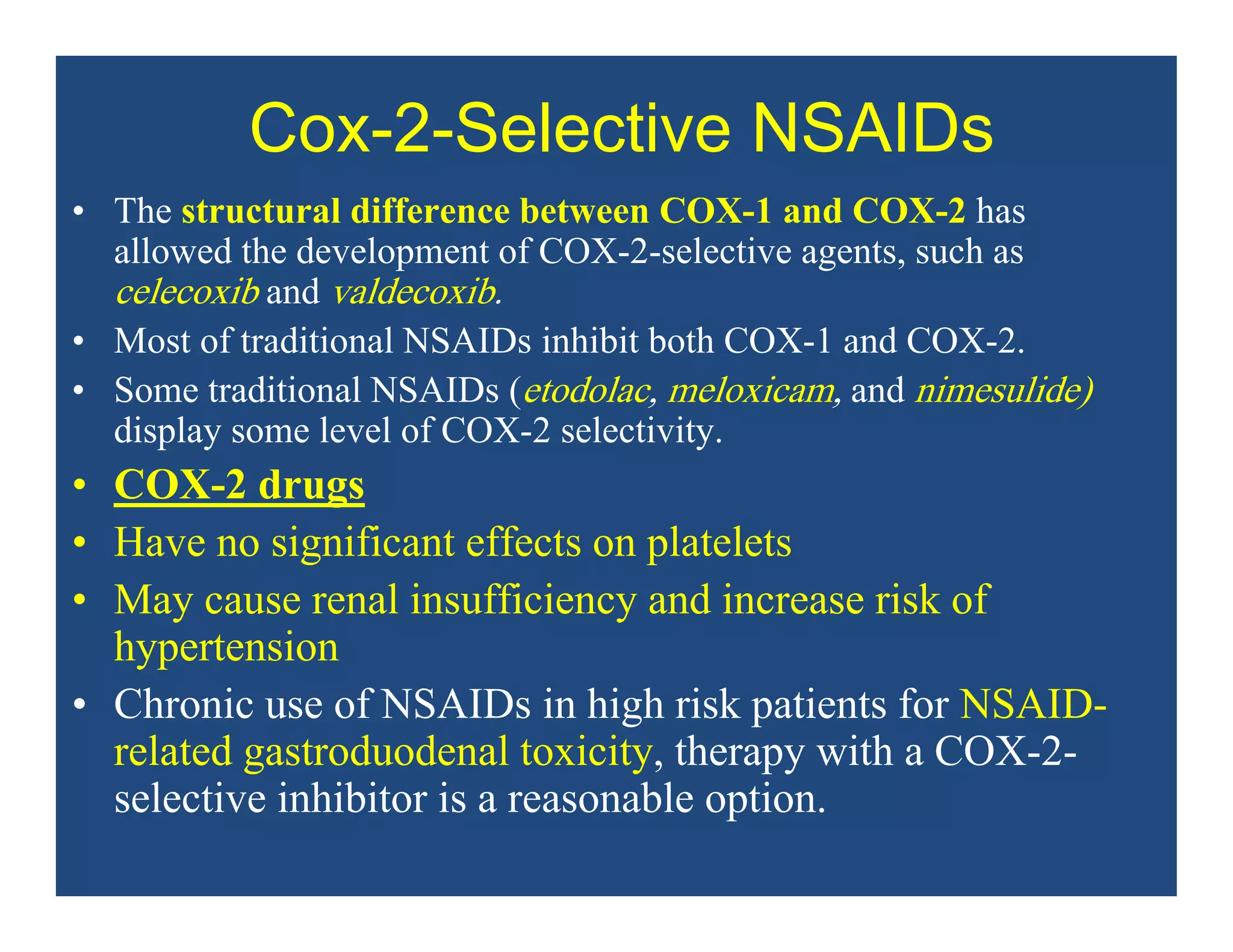
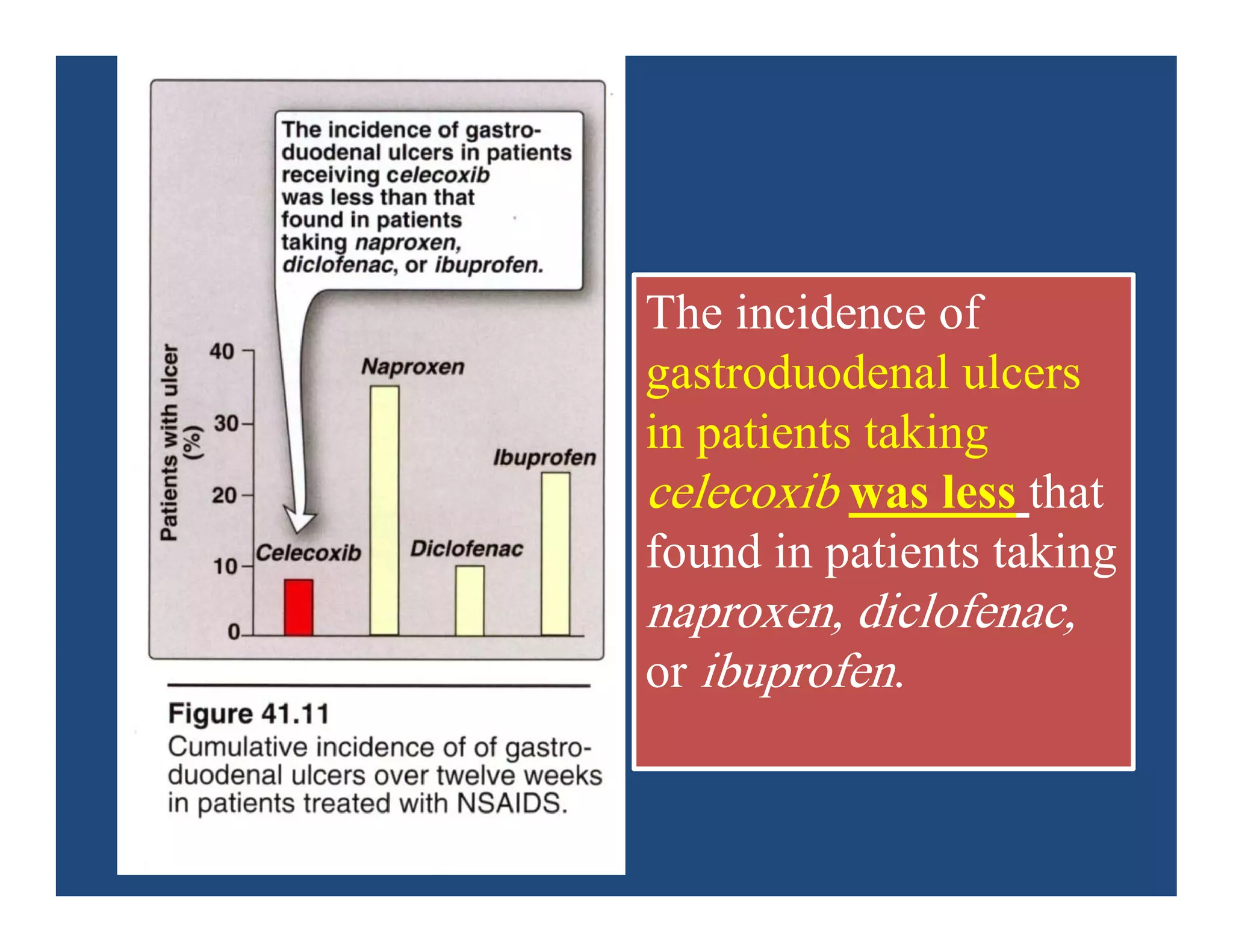
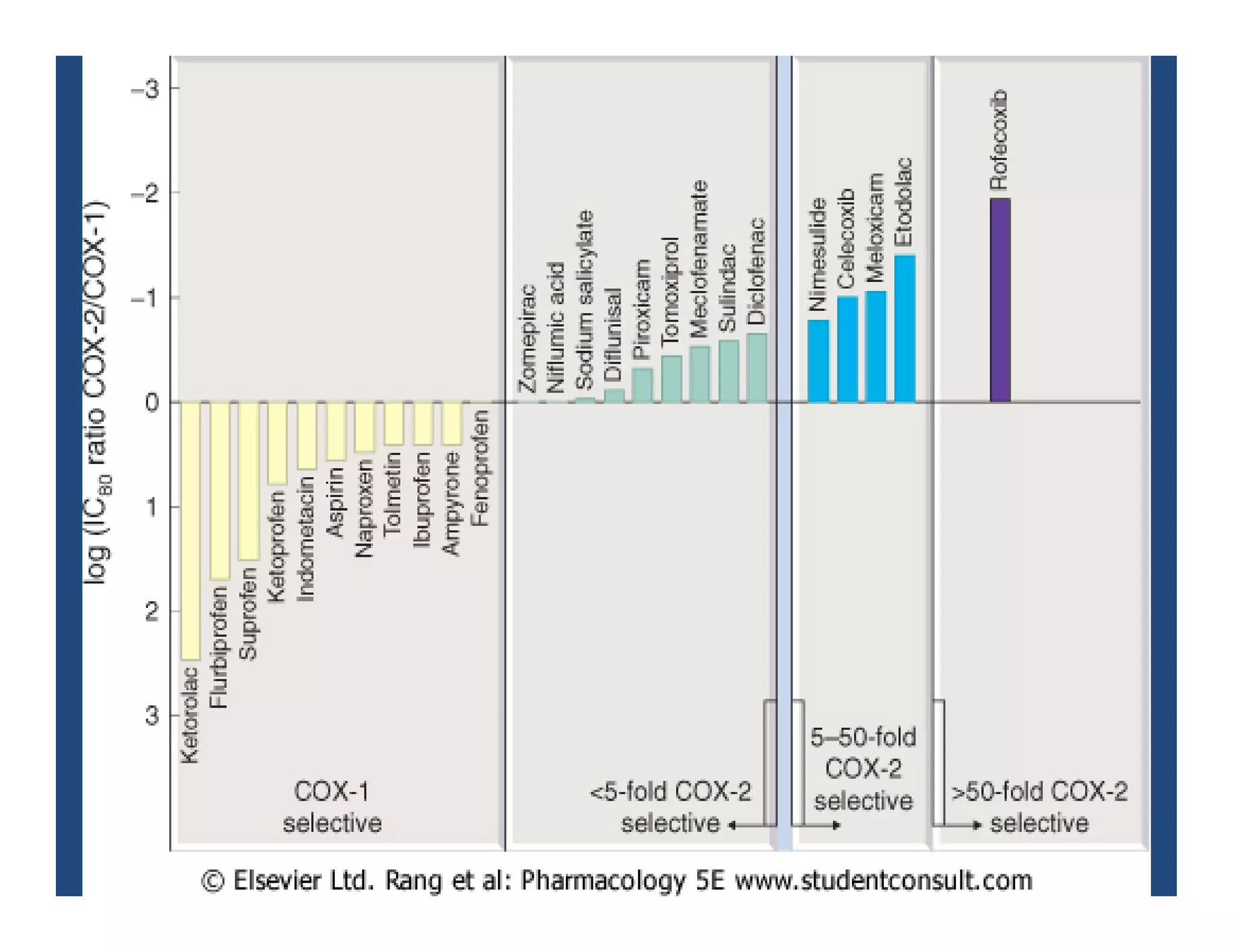
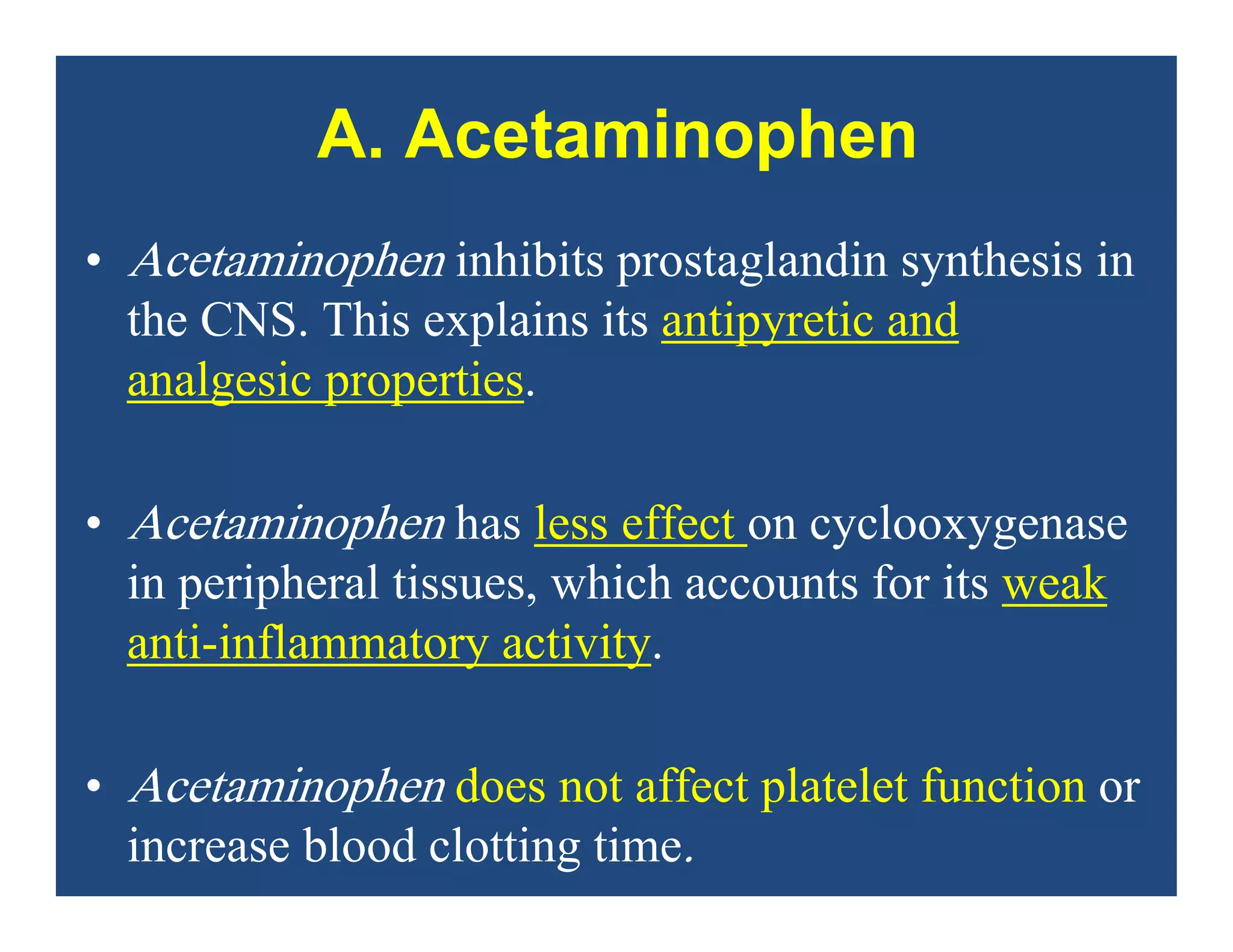
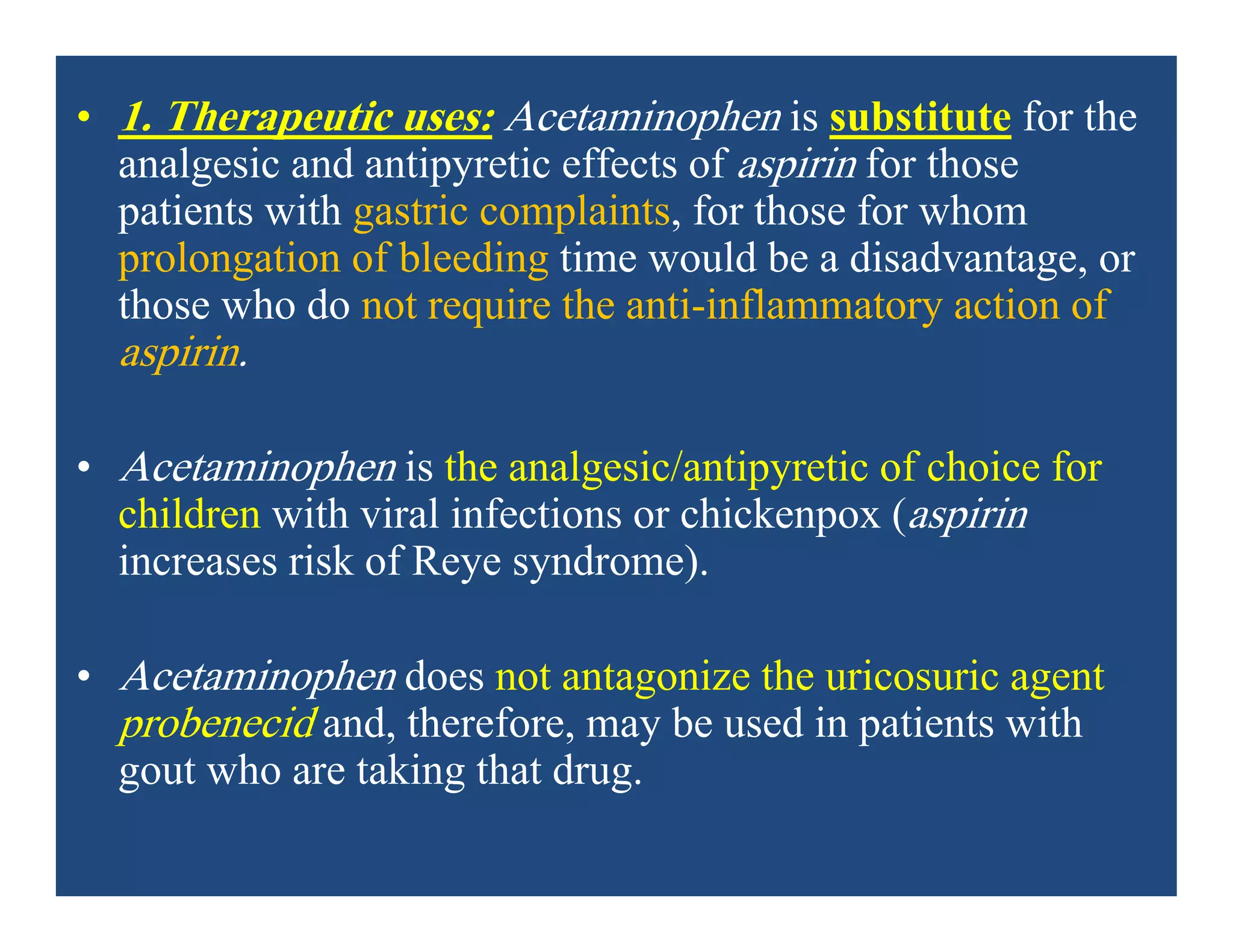
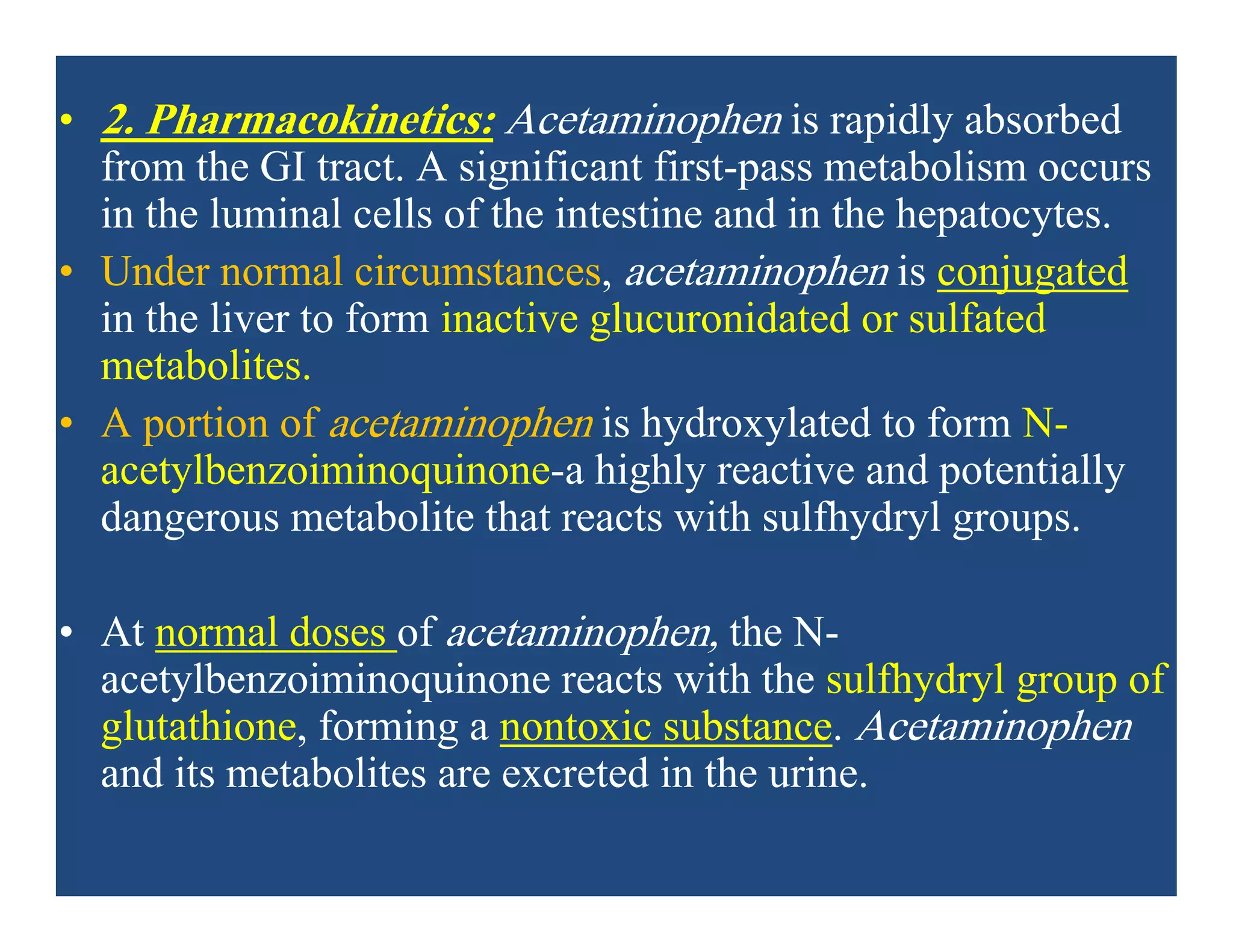
Inflammation is the body's natural response to injury or infection and involves increased blood flow, immune cell activity, and pain. Nonsteroidal anti-inflammatory drugs (NSAsIDs) like aspirin work by inhibiting the cyclooxygenase enzymes, decreasing the production of prostaglandins which mediate inflammation. Aspirin is unique in that it irreversibly inhibits cyclooxygenase. At normal doses, aspirin is hydrolyzed to salicylate which has analgesic, antipyretic, and anti-inflammatory effects. However, NSAIDs can cause adverse gastrointestinal, platelet, renal, and respiratory effects that require consideration of risks and benefits of long-term use.













![5. Adverse effects
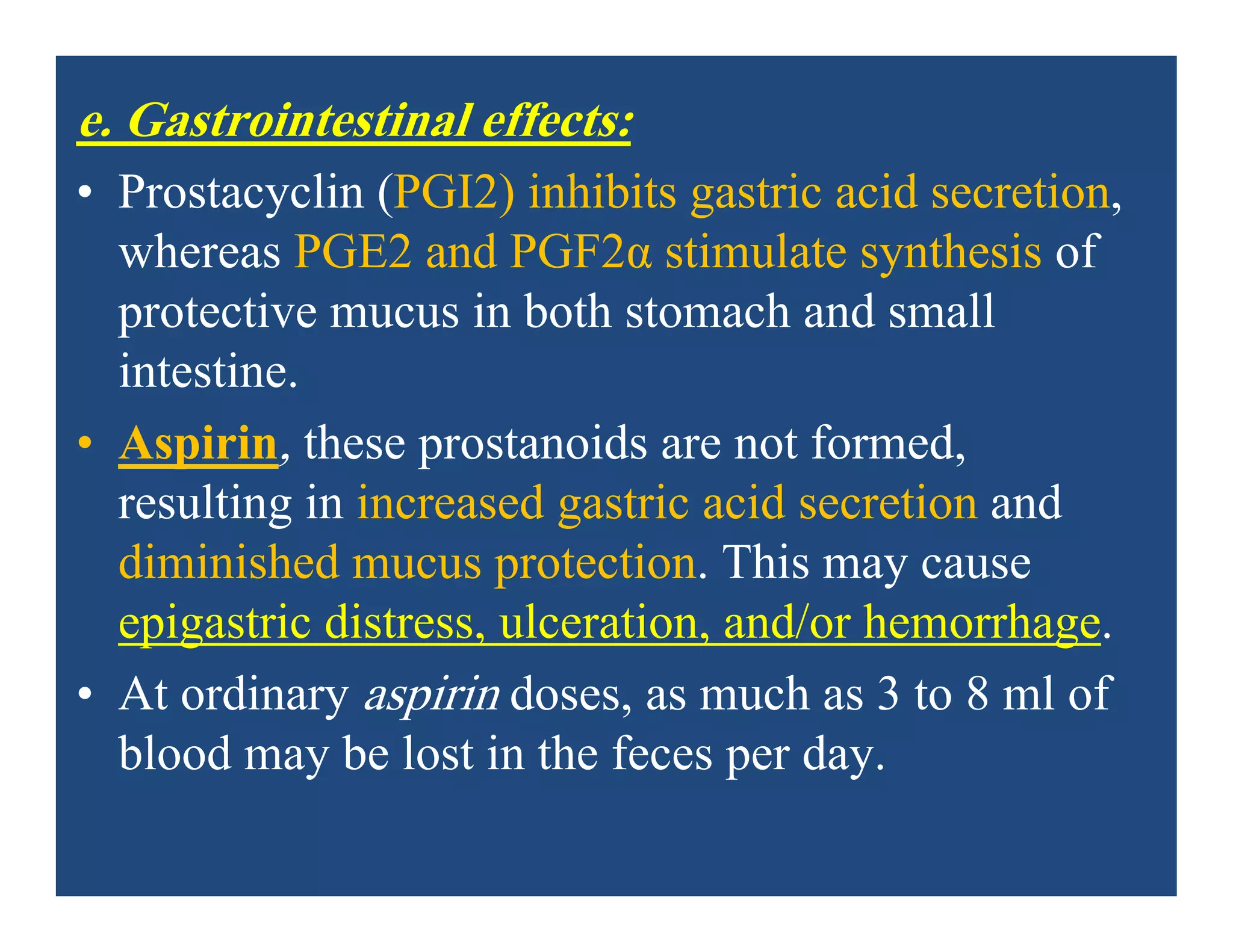
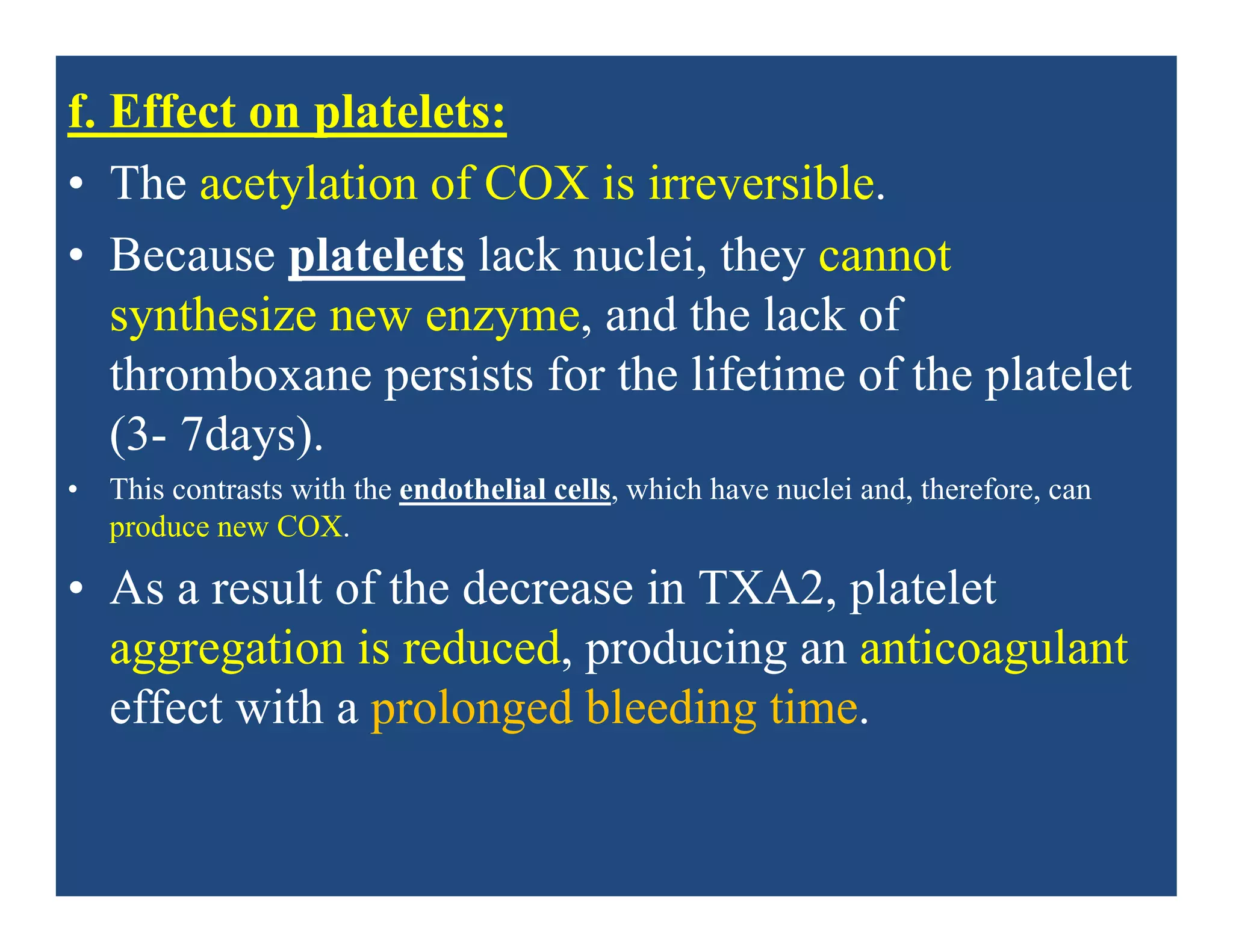
• a. Gastrointestinal: The most common gastrointestinal (GI) effects
of the salicylates are epigastric distress, nausea, and vomiting.
Microscopic GI bleeding is almost universal in patients treated with
salicylates.
• Aspirin is an acid. At stomach pH, aspirin is uncharged;
consequently, it readily crosses into mucosal cells, where it ionizes
(becomes negatively charged) and becomes trapped, thus potentially
causing direct damage to the cells. Aspirin should be taken with
food and large volumes of fluids to diminish GI disturbances.
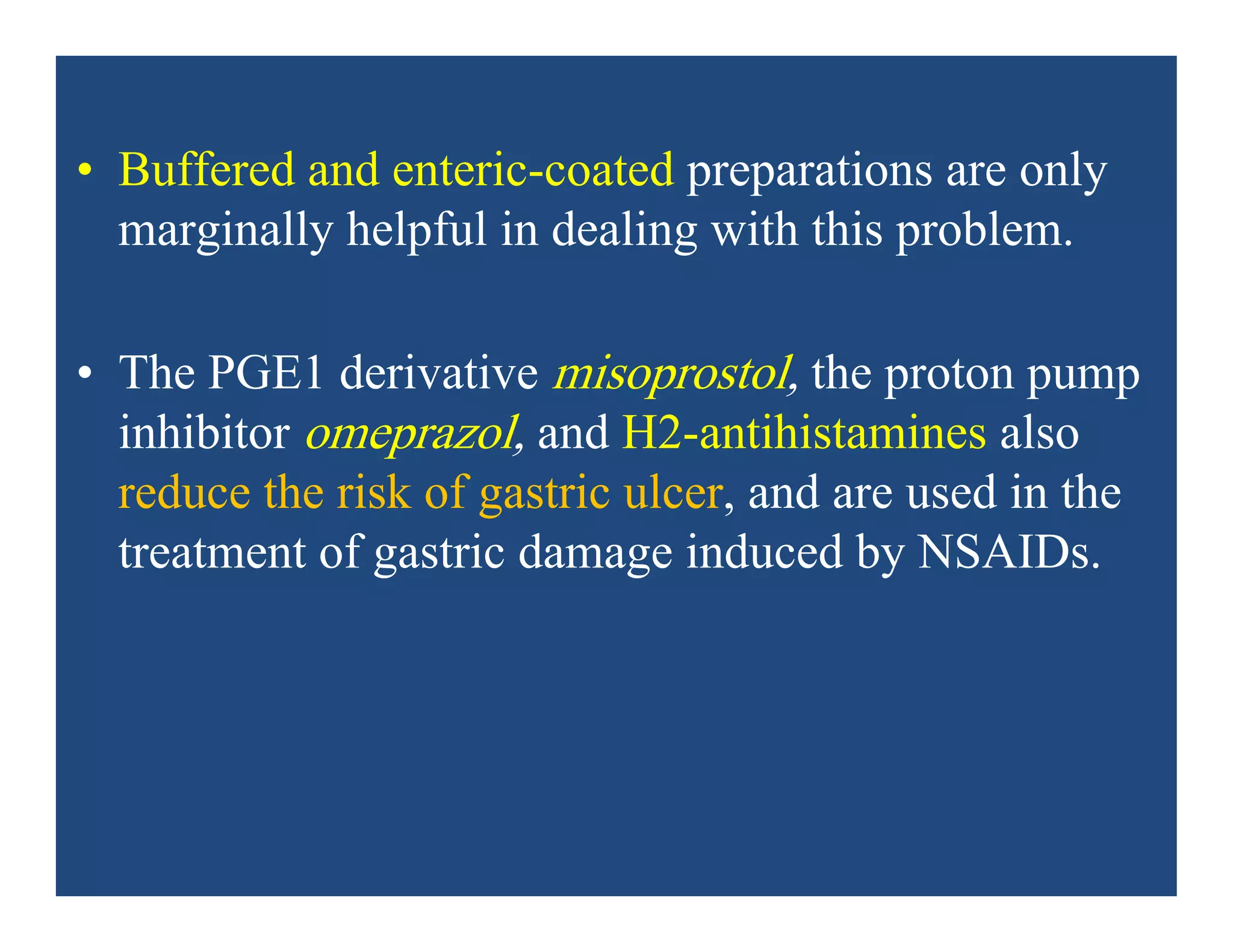
Alternatively, misoprostol may be taken concurrently.]](https://image.slidesharecdn.com/nsaids2014pdf-140718013250-phpapp02/75/NSAIDs-2014-40-2048.jpg)








![N-acetylcysteine
DOSAGE FORMS: Antidote, Mucolytic Agent
• Injection, solution:
• Acetadote®: 20% [200 mg/mL] (30 mL)
• Solution, inhalation/oral: 10% [100 mg/mL];
20% [200 mg/mL](https://image.slidesharecdn.com/nsaids2014pdf-140718013250-phpapp02/75/NSAIDs-2014-60-2048.jpg)